Severe Asthma Treatment Drugs Market Insights 2025, Analysis and Forecast to 2030, by Manufacturers, Regions, Technology, Product Type
- Single User License (1 Users) $ 3,600
- Team License (2~5 Users) $ 4,600
- Corporate License (>5 Users) $ 5,600
The severe asthma treatment drugs market constitutes a dynamic subset of the respiratory therapeutics arena, concentrating on biologic modulators that target underlying inflammatory pathways in patients unresponsive to standard inhaled corticosteroids and long-acting beta-agonists. Severe asthma, affecting 5-10% of the estimated 300 million global asthma cases, manifests as frequent exacerbations, persistent symptoms, and impaired lung function, often driven by type 2 inflammation involving eosinophils, IgE, or IL-5, or non-type 2 pathways like TSLP. The market's defining traits include monoclonal antibodies administered via subcutaneous injections every two to eight weeks, enabling personalized therapy based on biomarkers such as blood eosinophil counts, fractional exhaled nitric oxide, or serum IgE levels, which guide phenotyping for optimal drug selection. This precision approach has reduced oral corticosteroid dependence by up to 70% in responders, minimizing side effects like osteoporosis and diabetes, while fostering a shift from reactive exacerbation management to proactive disease control. Innovation emphasizes broad-spectrum agents like TSLP inhibitors that address both eosinophilic and non-eosinophilic endotypes, alongside self-injection devices enhancing patient autonomy and adherence rates above 80%. The sector contends with high annual costs exceeding $30,000 per patient, diagnostic underutilization in primary care, and the need for multidisciplinary specialist referrals, yet benefits from guideline updates from bodies like the Global Initiative for Asthma advocating early biologic initiation post-failure of high-dose inhalers. Real-world evidence underscores sustained reductions in emergency visits by 50-60%, with combination regimens exploring add-ons like macrolides for persistent cases. By 2025, the global severe asthma treatment drugs market is estimated to be valued between USD 28 billion and USD 38 billion, with a projected compound annual growth rate (CAGR) of 2% to 4% through 2030. This conservative trajectory aligns with maturing biologic penetration stabilizing at 40-50% of eligible patients, label expansions into adolescent populations, and payer-driven value-based agreements tying reimbursements to exacerbation-free intervals, offset by biosimilar introductions eroding originator premiums.
Regional Market Trends
The severe asthma treatment drugs market reveals divergent growth vectors across regions, modulated by prevalence rates, specialist density, and reimbursement maturity.
● North America: With a CAGR of 1.5%–3.5%, the United States commands the lion's share through widespread biomarker testing in over 70% of referrals and Medicare expansions covering self-pay injectables, where urban centers like New York and Los Angeles report 20% annual uptake increases amid tele-pulmonology integrations.
● Europe: Projected at a CAGR of 1.0%–3.0%, the United Kingdom and Germany lead via national health service formularies prioritizing cost-effectiveness analyses, with trends toward decentralized administration in community clinics to alleviate hospital burdens.
● Asia-Pacific: Growing at a CAGR of 2.5%–4.5%, Japan and China propel dynamics through rising urbanization exacerbating pollution-linked severe cases, supported by national insurance inclusions for eosinophil-targeted therapies in high-burden provinces.
● Latin America: At a CAGR of 2.0%–4.0%, Brazil and Mexico advance with public health campaigns targeting indigenous asthma hotspots, emphasizing affordable biosimilars to bridge access gaps in rural telemedicine voids.
● Middle East and Africa (MEA): Exhibiting a CAGR of 1.5%–3.5%, Saudi Arabia drives adoption via Vision 2030-funded allergy centers, though sub-Saharan Africa's underdiagnosis rates above 80% constrain progress despite WHO-backed screening pilots.
Type Analysis
The severe asthma treatment drugs market is delineated by type, featuring anti-cytokine monoclonals with distinct mechanisms and trajectories toward broader indications and reduced dosing frequencies.
● Dupilumab: Targeting IL-4 and IL-13 signaling, dupilumab (DUPIXENT by Regeneron/Sanofi) excels in broad type 2 inflammation, demonstrating 60% exacerbation reductions in trials; trends include pediatric extensions down to six months and combinations with PDE4 inhibitors for chronic rhinosinusitis comorbidities.
● Omalizumab: An anti-IgE antibody (XOLAIR by Roche/Novartis), omalizumab binds free IgE to prevent mast cell degranulation, favored for allergic phenotypes with 45% asthma control improvements; developments focus on fixed-dose regimens minimizing weight-based calculations and expansions into food allergy adjuncts.
● Mepolizumab: As an anti-IL-5 monoclonal (Nucala by GlaxoSmithKline), mepolizumab depletes eosinophils, achieving 50% oral steroid sparing in severe eosinophilic cases; current evolutions emphasize 100 mg monthly dosing optimizations and real-world registries validating long-term safety beyond five years.
● Benralizumab: This afucosylated anti-IL-5 receptor agent (FASENRA by AstraZeneca) induces rapid eosinophil apoptosis via enhanced ADCC, offering q8-week maintenance post-loading; trends highlight biomarker-agnostic use in non-eosinophilic subsets and head-to-head superiority data versus mepolizumab in exacerbation rates.
● Tezepelumab: A TSLP blocker (TEZSPIRE by Amgen/AstraZeneca), tezepelumab addresses upstream inflammation irrespective of type 2 status, with 56% relative risk reductions in severe uncontrolled asthma; emerging patterns involve adolescent approvals and exploratory pairings with JAK inhibitors for steroid-refractory patients.
● Others: Encompassing emerging anti-IL-33 or reslizumab, this category trends toward oral small molecules disrupting alarmin pathways, promising daily compliance without injections for early severe disease interception.
Company Profiles
● Regeneron/Sanofi: The duo's DUPIXENT (dupilumab) dominates with 2024 global net sales of USD 14-20 billion, propelled by 22% year-over-year growth in Q2 2025 alone, reflecting expansions into eosinophilic esophagitis and robust U.S. reimbursement. Sanofi's immunology franchise, including Regeneron collaborations, underpinned FY 2024 total sales of €41.1 billion, up 11.3% at CER.
● Amgen/AstraZeneca: Their TEZSPIRE (tezepelumab-ekko) garnered USD 1.5-2.0 billion in combined 2024 revenues, integrated into AstraZeneca's respiratory portfolio contributing to FY 2024 total revenues of $54.1 billion, up 21%. Amgen's collaboration revenue supported full-year 2024 totals of $33.4 billion, with 19% growth.
● AstraZeneca: AstraZeneca's FASENRA (benralizumab) achieved USD 1.5-2.0 billion in 2024 sales, up 12% and sustaining IL-5 leadership within its $54.1 billion FY 2024 revenue base.
● Roche/Novartis: XOLAIR (omalizumab) generated USD 3-4 billion in aggregate 2024 revenues, with Roche reporting U.S. sales of CHF 2.47 billion (~USD 2.9 billion) amid global expansions; Roche's total 2024 sales rose 7% to CHF levels supporting CHF 9.2 billion net income. Novartis's immunology contributions, including XOLAIR profit-sharing, aided 2024 net sales growth.
● GlaxoSmithKline: GSK's Nucala (mepolizumab) posted USD 2-2.5 billion in 2024 revenues, fueling specialty medicines within FY 2024 total sales of £31.4 billion, up 8%.
● Viatris/Sandoz/Teva Pharmaceuticals: These generics leaders focus on biosimilar pipelines for omalizumab and mepolizumab, enhancing affordability in emerging markets.
Industry Value Chain Analysis
The severe asthma treatment drugs value chain orchestrates from cytokine pathway elucidation to patient-centric delivery, emblematic of biologic complexity and biomarker integration. It initiates with R&D, leveraging transcriptomics to map endotypes and CRISPR-edited airway models for efficacy screening, culminating in Phase IIIb trials with composite endpoints like annualized exacerbation rates and quality-of-life scores, often under FDA breakthrough therapy designations accelerating approvals by 6-12 months. Manufacturing hinges on CHO cell fermentation for high-titer monoclonals, with downstream purification ensuring <1% host cell protein impurities via chromatography and viral inactivation, frequently outsourced to CDMOs for 500-1,000 liter scales amid demand surges. Supply chains mandate cold-chain validation for 2-8°C stability, incorporating blockchain traceability to avert shortages during flu seasons, with serialization per EU FMD and U.S. DSCSA. Regulatory navigation encompasses EMA scientific advice on pediatric extrapolations and post-approval pharmacovigilance via registries like the Severe Asthma Research Program monitoring hypersensitivity risks. Marketing deploys digital platforms and KOL symposia disseminating real-world data on steroid reductions, channeled through specialty pharmacies with copay accumulators capping patient costs at $0-100 monthly. End-user ecosystems involve pulmonologist-led clinics with spirometry integration and apps for adherence reminders, fostering shared decision-making. Collaborative models like Regeneron-Sanofi exemplify vertical integration from discovery to commercialization, optimizing royalties, while biosimilar entrants like Sandoz streamline analytical similarity dossiers, collectively amortizing $2-3 billion per asset development amid payer demands for health economic models demonstrating $10,000-20,000 per exacerbation avoided.
Opportunities and Challenges
Opportunities:
● Biomarker Advancements: AI-augmented phenotyping could identify 20-30% more eligible patients, particularly in Asia-Pacific, amplifying uptake via point-of-care tests integrated into primary care workflows.
● Pediatric and Adolescent Expansions: Label extensions for under-12 cohorts promise 15-20% volume growth, with school-based administration pilots in North America enhancing compliance.
● Combination Paradigms: Dual biologic regimens targeting TSLP and IL-5 could yield 70% remission rates in refractory cases, unlocking premium pricing through superiority trials.
● Biosimilar Accessibility: Affordable omalizumab alternatives in Latin America and MEA could triple penetration, supported by WHO prequalification for low-resource settings.
Challenges:
● Diagnostic Inertia: Only 30-40% of severe cases receive specialist evaluation, delaying biologics by 2-3 years and inflating indirect costs from uncontrolled exacerbations.
● Adherence Hurdles: Injection fatigue erodes 20% of discontinuations annually, necessitating user-friendly autoinjectors and telehealth support to sustain 85% persistence.
● Payer Pressures: Outcomes-based contracts tying rebates to exacerbation thresholds strain margins, especially with U.S. Medicare negotiations post-Inflation Reduction Act.
● Endotype Heterogeneity: Non-responders to type 2 agents, comprising 30%, demand costly switch trials, complicating guideline adherence in resource-limited Europe.
Chapter 1 Executive Summary
Chapter 2 Abbreviation and Acronyms
Chapter 3 Preface
3.1 Research Scope
3.2 Research Sources
3.2.1 Data Sources
3.2.2 Assumptions
3.3 Research Method
Chapter 4 Market Landscape
4.1 Market Overview
4.2 Classification/Types
4.3 Application/End Users
Chapter 5 Market Trend Analysis
5.1 introduction
5.2 Drivers
5.3 Restraints
5.4 Opportunities
5.5 Threats
Chapter 6 industry Chain Analysis
6.1 Upstream/Suppliers Analysis
6.2 Severe Asthma Treatment Drugs Analysis
6.2.1 Technology Analysis
6.2.2 Cost Analysis
6.2.3 Market Channel Analysis
6.3 Downstream Buyers/End Users
Chapter 7 Latest Market Dynamics
7.1 Latest News
7.2 Merger and Acquisition
7.3 Planned/Future Project
7.4 Policy Dynamics
Chapter 8 Historical and Forecast Severe Asthma Treatment Drugs Market in North America (2020-2030)
8.1 Severe Asthma Treatment Drugs Market Size
8.2 Severe Asthma Treatment Drugs Market by End Use
8.3 Competition by Players/Suppliers
8.4 Severe Asthma Treatment Drugs Market Size by Type
8.5 Key Countries Analysis
8.5.1 United States
8.5.2 Canada
8.5.3 Mexico
Chapter 9 Historical and Forecast Severe Asthma Treatment Drugs Market in South America (2020-2030)
9.1 Severe Asthma Treatment Drugs Market Size
9.2 Severe Asthma Treatment Drugs Market by End Use
9.3 Competition by Players/Suppliers
9.4 Severe Asthma Treatment Drugs Market Size by Type
9.5 Key Countries Analysis
9.5.1 Brazil
9.5.2 Argentina
9.5.3 Chile
9.5.4 Peru
Chapter 10 Historical and Forecast Severe Asthma Treatment Drugs Market in Asia & Pacific (2020-2030)
10.1 Severe Asthma Treatment Drugs Market Size
10.2 Severe Asthma Treatment Drugs Market by End Use
10.3 Competition by Players/Suppliers
10.4 Severe Asthma Treatment Drugs Market Size by Type
10.5 Key Countries Analysis
10.5.1 China
10.5.2 India
10.5.3 Japan
10.5.4 South Korea
10.5.5 Southest Asia
10.5.6 Australia
Chapter 11 Historical and Forecast Severe Asthma Treatment Drugs Market in Europe (2020-2030)
11.1 Severe Asthma Treatment Drugs Market Size
11.2 Severe Asthma Treatment Drugs Market by End Use
11.3 Competition by Players/Suppliers
11.4 Severe Asthma Treatment Drugs Market Size by Type
11.5 Key Countries Analysis
11.5.1 Germany
11.5.2 France
11.5.3 United Kingdom
11.5.4 Italy
11.5.5 Spain
11.5.6 Belgium
11.5.7 Netherlands
11.5.8 Austria
11.5.9 Poland
11.5.10 Russia
Chapter 12 Historical and Forecast Severe Asthma Treatment Drugs Market in MEA (2020-2030)
12.1 Severe Asthma Treatment Drugs Market Size
12.2 Severe Asthma Treatment Drugs Market by End Use
12.3 Competition by Players/Suppliers
12.4 Severe Asthma Treatment Drugs Market Size by Type
12.5 Key Countries Analysis
12.5.1 Egypt
12.5.2 Israel
12.5.3 South Africa
12.5.4 Gulf Cooperation Council Countries
12.5.5 Turkey
Chapter 13 Summary For Global Severe Asthma Treatment Drugs Market (2020-2025)
13.1 Severe Asthma Treatment Drugs Market Size
13.2 Severe Asthma Treatment Drugs Market by End Use
13.3 Competition by Players/Suppliers
13.4 Severe Asthma Treatment Drugs Market Size by Type
Chapter 14 Global Severe Asthma Treatment Drugs Market Forecast (2025-2030)
14.1 Severe Asthma Treatment Drugs Market Size Forecast
14.2 Severe Asthma Treatment Drugs Application Forecast
14.3 Competition by Players/Suppliers
14.4 Severe Asthma Treatment Drugs Type Forecast
Chapter 15 Analysis of Global Key Vendors
15.1 Regeneron
15.1.1 Company Profile
15.1.2 Main Business and Severe Asthma Treatment Drugs Information
15.1.3 SWOT Analysis of Regeneron
15.1.4 Regeneron Severe Asthma Treatment Drugs Sales, Revenue, Price and Gross Margin (2020-2025)
15.2 Sanofi
15.2.1 Company Profile
15.2.2 Main Business and Severe Asthma Treatment Drugs Information
15.2.3 SWOT Analysis of Sanofi
15.2.4 Sanofi Severe Asthma Treatment Drugs Sales, Revenue, Price and Gross Margin (2020-2025)
15.3 Roche
15.3.1 Company Profile
15.3.2 Main Business and Severe Asthma Treatment Drugs Information
15.3.3 SWOT Analysis of Roche
15.3.4 Roche Severe Asthma Treatment Drugs Sales, Revenue, Price and Gross Margin (2020-2025)
15.4 Novartis
15.4.1 Company Profile
15.4.2 Main Business and Severe Asthma Treatment Drugs Information
15.4.3 SWOT Analysis of Novartis
15.4.4 Novartis Severe Asthma Treatment Drugs Sales, Revenue, Price and Gross Margin (2020-2025)
15.5 GlaxoSmithKline
15.5.1 Company Profile
15.5.2 Main Business and Severe Asthma Treatment Drugs Information
15.5.3 SWOT Analysis of GlaxoSmithKline
15.5.4 GlaxoSmithKline Severe Asthma Treatment Drugs Sales, Revenue, Price and Gross Margin (2020-2025)
15.6 AstraZeneca
15.6.1 Company Profile
15.6.2 Main Business and Severe Asthma Treatment Drugs Information
15.6.3 SWOT Analysis of AstraZeneca
15.6.4 AstraZeneca Severe Asthma Treatment Drugs Sales, Revenue, Price and Gross Margin (2020-2025)
15.7 Amgen
15.7.1 Company Profile
15.7.2 Main Business and Severe Asthma Treatment Drugs Information
15.7.3 SWOT Analysis of Amgen
15.7.4 Amgen Severe Asthma Treatment Drugs Sales, Revenue, Price and Gross Margin (2020-2025)
15.8 Viatris
15.8.1 Company Profile
15.8.2 Main Business and Severe Asthma Treatment Drugs Information
15.8.3 SWOT Analysis of Viatris
15.8.4 Viatris Severe Asthma Treatment Drugs Sales, Revenue, Price and Gross Margin (2020-2025)
15.9 Sandoz
15.9.1 Company Profile
15.9.2 Main Business and Severe Asthma Treatment Drugs Information
15.9.3 SWOT Analysis of Sandoz
15.9.4 Sandoz Severe Asthma Treatment Drugs Sales, Revenue, Price and Gross Margin (2020-2025)
15.10 Teva Pharmaceuticals
15.10.1 Company Profile
15.10.2 Main Business and Severe Asthma Treatment Drugs Information
15.10.3 SWOT Analysis of Teva Pharmaceuticals
15.10.4 Teva Pharmaceuticals Severe Asthma Treatment Drugs Sales, Revenue, Price and Gross Margin (2020-2025)
Please ask for sample pages for full companies list
Table Research Scope of Severe Asthma Treatment Drugs Report
Table Data Sources of Severe Asthma Treatment Drugs Report
Table Major Assumptions of Severe Asthma Treatment Drugs Report
Table Severe Asthma Treatment Drugs Classification
Table Severe Asthma Treatment Drugs Applications
Table Drivers of Severe Asthma Treatment Drugs Market
Table Restraints of Severe Asthma Treatment Drugs Market
Table Opportunities of Severe Asthma Treatment Drugs Market
Table Threats of Severe Asthma Treatment Drugs Market
Table Raw Materials Suppliers
Table Different Production Methods of Severe Asthma Treatment Drugs
Table Cost Structure Analysis of Severe Asthma Treatment Drugs
Table Key End Users
Table Latest News of Severe Asthma Treatment Drugs Market
Table Merger and Acquisition
Table Planned/Future Project of Severe Asthma Treatment Drugs Market
Table Policy of Severe Asthma Treatment Drugs Market
Table 2020-2030 North America Severe Asthma Treatment Drugs Market Size
Table 2020-2030 North America Severe Asthma Treatment Drugs Market Size by Application
Table 2020-2025 North America Severe Asthma Treatment Drugs Key Players Revenue
Table 2020-2025 North America Severe Asthma Treatment Drugs Key Players Market Share
Table 2020-2030 North America Severe Asthma Treatment Drugs Market Size by Type
Table 2020-2030 United States Severe Asthma Treatment Drugs Market Size
Table 2020-2030 Canada Severe Asthma Treatment Drugs Market Size
Table 2020-2030 Mexico Severe Asthma Treatment Drugs Market Size
Table 2020-2030 South America Severe Asthma Treatment Drugs Market Size
Table 2020-2030 South America Severe Asthma Treatment Drugs Market Size by Application
Table 2020-2025 South America Severe Asthma Treatment Drugs Key Players Revenue
Table 2020-2025 South America Severe Asthma Treatment Drugs Key Players Market Share
Table 2020-2030 South America Severe Asthma Treatment Drugs Market Size by Type
Table 2020-2030 Brazil Severe Asthma Treatment Drugs Market Size
Table 2020-2030 Argentina Severe Asthma Treatment Drugs Market Size
Table 2020-2030 Chile Severe Asthma Treatment Drugs Market Size
Table 2020-2030 Peru Severe Asthma Treatment Drugs Market Size
Table 2020-2030 Asia & Pacific Severe Asthma Treatment Drugs Market Size
Table 2020-2030 Asia & Pacific Severe Asthma Treatment Drugs Market Size by Application
Table 2020-2025 Asia & Pacific Severe Asthma Treatment Drugs Key Players Revenue
Table 2020-2025 Asia & Pacific Severe Asthma Treatment Drugs Key Players Market Share
Table 2020-2030 Asia & Pacific Severe Asthma Treatment Drugs Market Size by Type
Table 2020-2030 China Severe Asthma Treatment Drugs Market Size
Table 2020-2030 India Severe Asthma Treatment Drugs Market Size
Table 2020-2030 Japan Severe Asthma Treatment Drugs Market Size
Table 2020-2030 South Korea Severe Asthma Treatment Drugs Market Size
Table 2020-2030 Southeast Asia Severe Asthma Treatment Drugs Market Size
Table 2020-2030 Australia Severe Asthma Treatment Drugs Market Size
Table 2020-2030 Europe Severe Asthma Treatment Drugs Market Size
Table 2020-2030 Europe Severe Asthma Treatment Drugs Market Size by Application
Table 2020-2025 Europe Severe Asthma Treatment Drugs Key Players Revenue
Table 2020-2025 Europe Severe Asthma Treatment Drugs Key Players Market Share
Table 2020-2030 Europe Severe Asthma Treatment Drugs Market Size by Type
Table 2020-2030 Germany Severe Asthma Treatment Drugs Market Size
Table 2020-2030 France Severe Asthma Treatment Drugs Market Size
Table 2020-2030 United Kingdom Severe Asthma Treatment Drugs Market Size
Table 2020-2030 Italy Severe Asthma Treatment Drugs Market Size
Table 2020-2030 Spain Severe Asthma Treatment Drugs Market Size
Table 2020-2030 Belgium Severe Asthma Treatment Drugs Market Size
Table 2020-2030 Netherlands Severe Asthma Treatment Drugs Market Size
Table 2020-2030 Austria Severe Asthma Treatment Drugs Market Size
Table 2020-2030 Poland Severe Asthma Treatment Drugs Market Size
Table 2020-2030 Russia Severe Asthma Treatment Drugs Market Size
Table 2020-2030 MEA Severe Asthma Treatment Drugs Market Size
Table 2020-2030 MEA Severe Asthma Treatment Drugs Market Size by Application
Table 2020-2025 MEA Severe Asthma Treatment Drugs Key Players Revenue
Table 2020-2025 MEA Severe Asthma Treatment Drugs Key Players Market Share
Table 2020-2030 MEA Severe Asthma Treatment Drugs Market Size by Type
Table 2020-2030 Egypt Severe Asthma Treatment Drugs Market Size
Table 2020-2030 Israel Severe Asthma Treatment Drugs Market Size
Table 2020-2030 South Africa Severe Asthma Treatment Drugs Market Size
Table 2020-2030 Gulf Cooperation Council Countries Severe Asthma Treatment Drugs Market Size
Table 2020-2030 Turkey Severe Asthma Treatment Drugs Market Size
Table 2020-2025 Global Severe Asthma Treatment Drugs Market Size by Region
Table 2020-2025 Global Severe Asthma Treatment Drugs Market Size Share by Region
Table 2020-2025 Global Severe Asthma Treatment Drugs Market Size by Application
Table 2020-2025 Global Severe Asthma Treatment Drugs Market Share by Application
Table 2020-2025 Global Severe Asthma Treatment Drugs Key Vendors Revenue
Table 2020-2025 Global Severe Asthma Treatment Drugs Key Vendors Market Share
Table 2020-2025 Global Severe Asthma Treatment Drugs Market Size by Type
Table 2020-2025 Global Severe Asthma Treatment Drugs Market Share by Type
Table 2025-2030 Global Severe Asthma Treatment Drugs Market Size by Region
Table 2025-2030 Global Severe Asthma Treatment Drugs Market Size Share by Region
Table 2025-2030 Global Severe Asthma Treatment Drugs Market Size by Application
Table 2025-2030 Global Severe Asthma Treatment Drugs Market Share by Application
Table 2025-2030 Global Severe Asthma Treatment Drugs Key Vendors Revenue
Table 2025-2030 Global Severe Asthma Treatment Drugs Key Vendors Market Share
Table 2025-2030 Global Severe Asthma Treatment Drugs Market Size by Type
Table 2025-2030 Severe Asthma Treatment Drugs Global Market Share by Type
Figure Market Size Estimated Method
Figure Major Forecasting Factors
Figure Severe Asthma Treatment Drugs Picture
Figure 2020-2030 North America Severe Asthma Treatment Drugs Market Size and CAGR
Figure 2020-2030 South America Severe Asthma Treatment Drugs Market Size and CAGR
Figure 2020-2030 Asia & Pacific Severe Asthma Treatment Drugs Market Size and CAGR
Figure 2020-2030 Europe Severe Asthma Treatment Drugs Market Size and CAGR
Figure 2020-2030 MEA Severe Asthma Treatment Drugs Market Size and CAGR
Figure 2020-2025 Global Severe Asthma Treatment Drugs Market Size and Growth Rate
Figure 2025-2030 Global Severe Asthma Treatment Drugs Market Size and Growth Rate
Research Methodology
- Market Estimated Methodology:
Bottom-up & top-down approach, supply & demand approach are the most important method which is used by HDIN Research to estimate the market size.
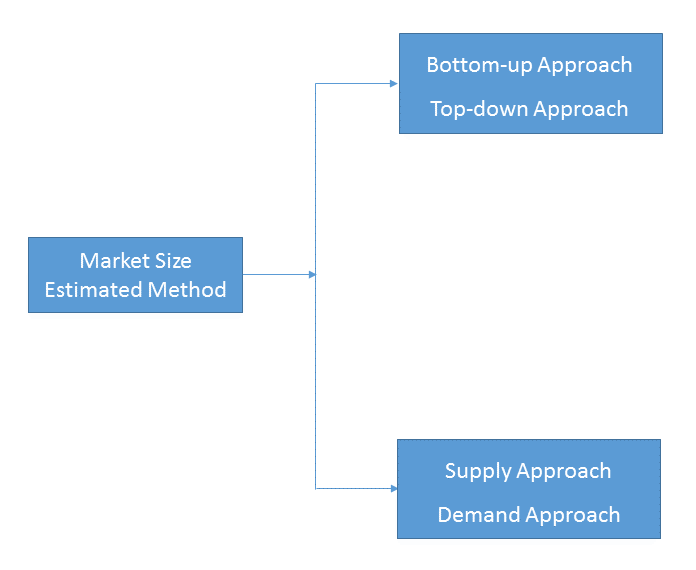
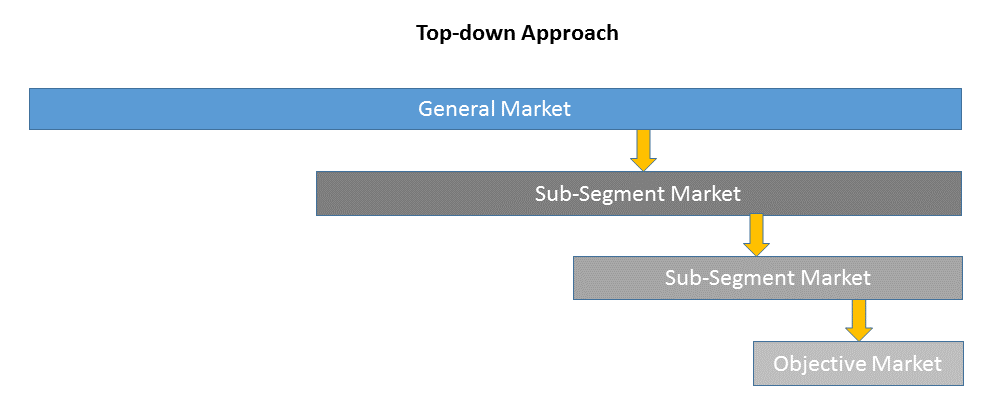
1)Top-down & Bottom-up Approach
Top-down approach uses a general market size figure and determines the percentage that the objective market represents.
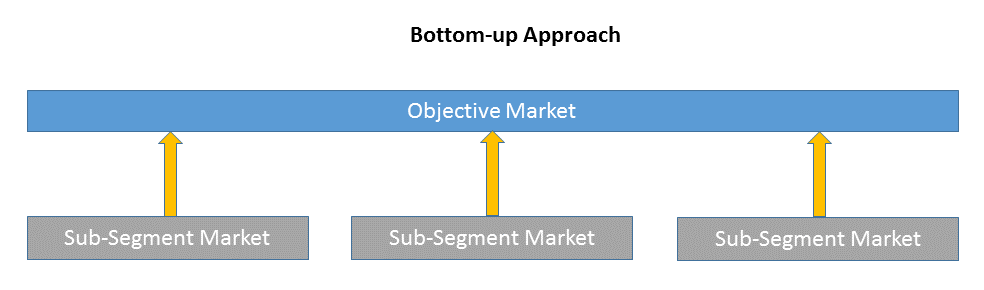
Bottom-up approach size the objective market by collecting the sub-segment information.
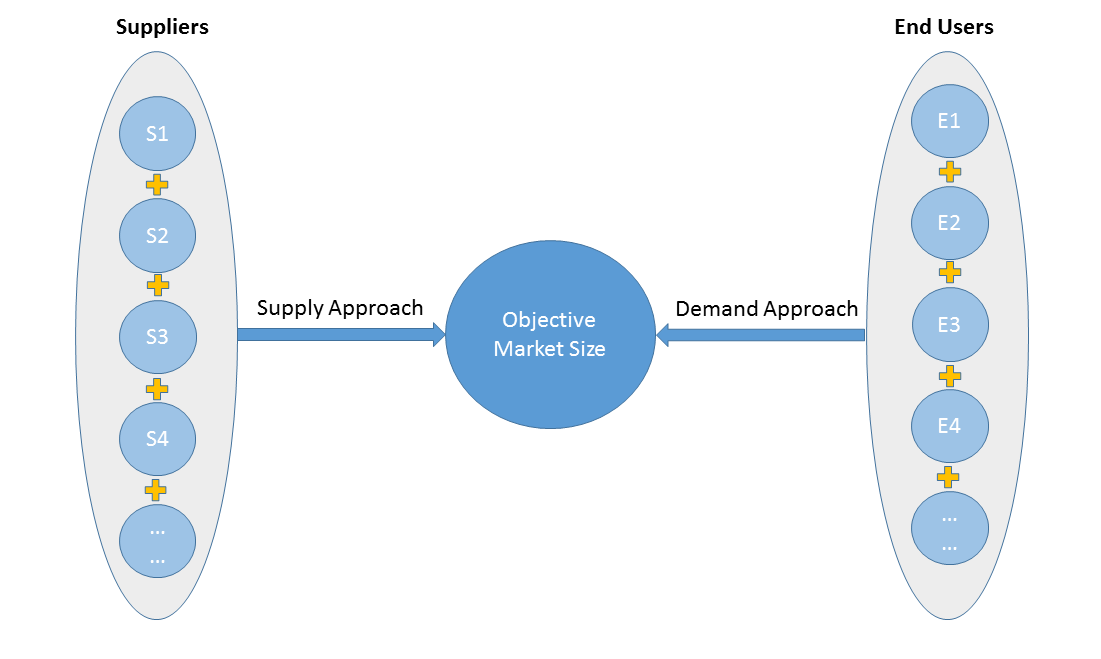
2)Supply & Demand Approach
Supply approach is based on assessments of the size of each competitor supplying the objective market.
Demand approach combine end-user data within a market to estimate the objective market size. It is sometimes referred to as bottom-up approach.
- Forecasting Methodology
- Numerous factors impacting the market trend are considered for forecast model:
- New technology and application in the future;
- New project planned/under contraction;
- Global and regional underlying economic growth;
- Threatens of substitute products;
- Industry expert opinion;
- Policy and Society implication.
- Analysis Tools
1)PEST Analysis
PEST Analysis is a simple and widely used tool that helps our client analyze the Political, Economic, Socio-Cultural, and Technological changes in their business environment.
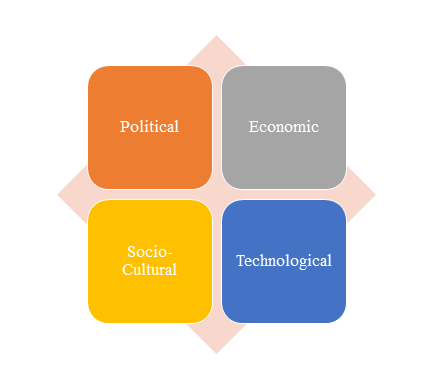
- Benefits of a PEST analysis:
- It helps you to spot business opportunities, and it gives you advanced warning of significant threats.
- It reveals the direction of change within your business environment. This helps you shape what you’re doing, so that you work with change, rather than against it.
- It helps you avoid starting projects that are likely to fail, for reasons beyond your control.
- It can help you break free of unconscious assumptions when you enter a new country, region, or market; because it helps you develop an objective view of this new environment.
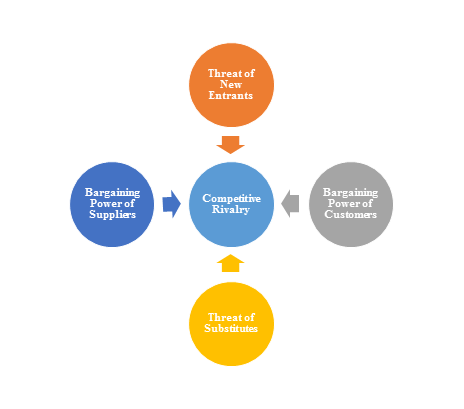
2)Porter’s Five Force Model Analysis
The Porter’s Five Force Model is a tool that can be used to analyze the opportunities and overall competitive advantage. The five forces that can assist in determining the competitive intensity and potential attractiveness within a specific area.
- Threat of New Entrants: Profitable industries that yield high returns will attract new firms.
- Threat of Substitutes: A substitute product uses a different technology to try to solve the same economic need.
- Bargaining Power of Customers: the ability of customers to put the firm under pressure, which also affects the customer's sensitivity to price changes.
- Bargaining Power of Suppliers: Suppliers of raw materials, components, labor, and services (such as expertise) to the firm can be a source of power over the firm when there are few substitutes.
- Competitive Rivalry: For most industries the intensity of competitive rivalry is the major determinant of the competitiveness of the industry.
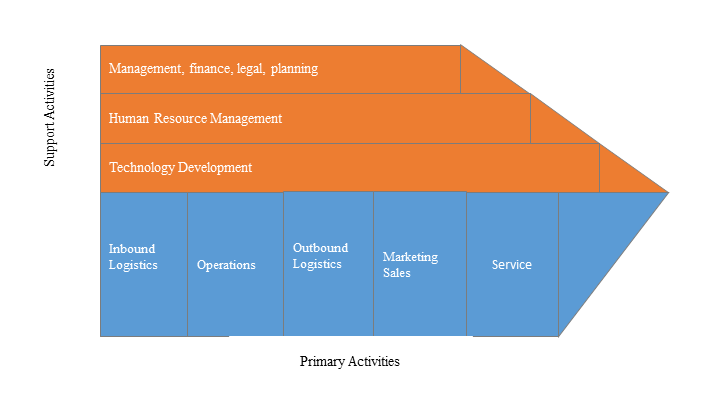
3)Value Chain Analysis
Value chain analysis is a tool to identify activities, within and around the firm and relating these activities to an assessment of competitive strength. Value chain can be analyzed by primary activities and supportive activities. Primary activities include: inbound logistics, operations, outbound logistics, marketing & sales, service. Support activities include: technology development, human resource management, management, finance, legal, planning.
4)SWOT Analysis
SWOT analysis is a tool used to evaluate a company's competitive position by identifying its strengths, weaknesses, opportunities and threats. The strengths and weakness is the inner factor; the opportunities and threats are the external factor. By analyzing the inner and external factors, the analysis can provide the detail information of the position of a player and the characteristics of the industry.
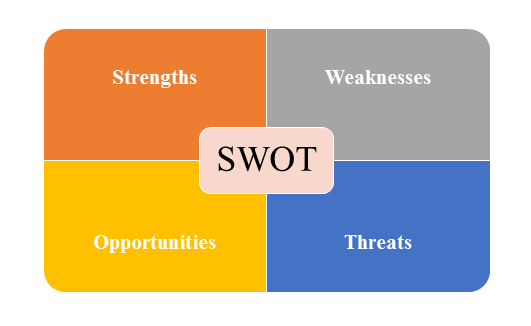
- Strengths describe what the player excels at and separates it from the competition
- Weaknesses stop the player from performing at its optimum level.
- Opportunities refer to favorable external factors that the player can use to give it a competitive advantage.
- Threats refer to factors that have the potential to harm the player.
- Data Sources
| Primary Sources | Secondary Sources |
|---|---|
| Face to face/Phone Interviews with market participants, such as: Manufactures; Distributors; End-users; Experts. Online Survey |
Government/International Organization Data: Annual Report/Presentation/Fact Book Internet Source Information Industry Association Data Free/Purchased Database Market Research Report Book/Journal/News |