Botulinum Toxin Injection Market Insights 2025, Analysis and Forecast to 2030, by Manufacturers, Regions, Technology, Product Type
- Single User License (1 Users) $ 3,600
- Team License (2~5 Users) $ 4,600
- Corporate License (>5 Users) $ 5,600
The botulinum toxin injection market represents a critical segment within the aesthetic medicine and therapeutic biologics landscape, characterized by its dual role in cosmetic enhancement and clinical treatment applications. Botulinum toxin is a biological agent comprising toxic proteins produced during the reproductive process of Clostridium botulinum bacteria. Based on antigenic properties, eight serotypes (A, B, Ca, Cb, D, E, F, G) exist, with Type A dominating aesthetic applications due to its efficacy and safety profile. The mechanism of action involves injection into targeted muscles, where the toxin acts on peripheral motor nerve endings at the neuromuscular junction and synaptic interface, inhibiting acetylcholine release from presynaptic membranes. This blockade reduces muscle tension or induces controlled paralysis, preventing localized muscle contraction and achieving aesthetic improvement. Effects are temporary, with metabolic degradation beginning after approximately one month, with muscle function gradually recovering over six to eight months, necessitating repeat treatments for sustained results. Botulinum toxin injection serves as an acetylcholine release inhibitor and neuromuscular blocking agent indicated across four primary aesthetic domains: temporary improvement of moderate to severe glabellar lines (frown lines between eyebrows), moderate to severe crow's feet, moderate to severe forehead lines in adults, and moderate to severe platysma bands. The global market achieved an estimated valuation between USD 5–8 billion in 2025, reflecting robust demand across cosmetic dermatology, plastic surgery, and medical aesthetics channels. Projections indicate sustained expansion at a compound annual growth rate (CAGR) spanning 5.5%–9.5% through 2030, driven by rising aesthetic consciousness, demographic aging, male grooming trends, and expanding indications beyond traditional facial rejuvenation. This growth trajectory reflects the convergence of technological refinement in formulation purity, injection techniques, and expanding geographic penetration into emerging markets where disposable income growth supports discretionary aesthetic spending. According to the International Society of Aesthetic Plastic Surgery (ISAPS) annual Global Survey released at the ISAPS Olympiad World Congress in Singapore, more than 17.4 million surgical procedures and 20.5 million non-surgical procedures were performed by plastic surgeons in 2024, representing an overall increase of 42.5% over four years. Botulinum toxin maintained its position as the most common non-surgical procedure for both men and women across all age groups, with 7.8 million procedures performed globally by plastic surgeons. This substantial procedure volume underscores the treatment's mainstream acceptance and integration into standard aesthetic practice. The United States led global procedure volumes with over 6.1 million treatments, followed by Brazil with 3.1 million (which ranked first in surgical procedures with 2.3 million) and Japan, highlighting geographic concentration in developed markets with established aesthetic medicine infrastructure.
● Type Analysis
The market bifurcates into two primary categories based on production methodology: natural botulinum toxin injections and recombinant botulinum toxin injections, each presenting distinct advantages in safety profiles, manufacturing control, and therapeutic consistency.
● Natural Botulinum Toxin Injection: Traditional formulations utilize toxin proteins expressed through bacterial fermentation of Clostridium botulinum strains. During bacterial proliferation, botulinum toxin spontaneously activates within the bacterial organism, producing highly toxic activated protein through an uncontrolled process. The resulting commercial product comprises a complex mixture of botulinum toxin protein and multiple accessory proteins, with the latter lacking defined therapeutic function but potentially stimulating immune system responses and antibody formation in recipients. While natural botulinum toxin maintains market dominance through established regulatory approvals, extensive clinical validation spanning decades, and surgeon familiarity, its production methodology carries inherent biological safety concerns related to pathogenic bacterial handling, batch-to-batch variability from inconsistent activation processes, and immunogenicity risks from heterogeneous protein composition.
● Recombinant Botulinum Toxin Injection: Representing a paradigm shift in production technology, recombinant variants demonstrate superior advantages in both safety and efficacy profiles. Recombinant production applies genetic engineering techniques to mimic natural botulinum toxin activation mechanisms while fundamentally altering the manufacturing paradigm. The process involves expressing inactive botulinum toxin protein in non-pathogenic engineered bacterial hosts, followed by fermentation expansion and subsequent extracellular activation of the toxin protein outside the bacterial system. This complete production cycle eliminates reliance on pathogenic Clostridium botulinum, with manufacturing workflows executed under strict quality control protocols, dramatically reducing biological safety risks. The removal of extraneous proteins yields highly purified single-toxin protein formulations, enhancing therapeutic efficacy consistency. Natural botulinum toxin complexes containing multiple accessory proteins without clear therapeutic roles may trigger immune responses and antibody development. Recombinant formulations, comprising pure singular botulinum toxin protein, minimize antibody formation risk while employing highly efficient, controlled processes ensuring exceptional batch-to-batch consistency, delivering therapeutically stable products for clinical application. Chongqing Yuyan Pharmaceutical Co. Ltd.'s YY001 represents a landmark advancement as the world's first and currently only recombinant Type A botulinum toxin product to receive regulatory submission. In 2024, YY001 was submitted to China's CFDA (now NMPA) for marketing authorization, seeking indication for temporary improvement of moderate to severe glabellar lines caused by corrugator and/or procerus muscle activity in adults aged 65 years and younger. This pioneering submission signals potential market disruption as recombinant technology addresses longstanding safety and consistency concerns inherent to natural formulations, potentially establishing new manufacturing standards and competitive benchmarks.
Regional Market Dynamics
● North America: The region maintains market leadership driven by the United States, where advanced aesthetic medicine ecosystems, high consumer spending power, and cultural acceptance of cosmetic procedures fuel sustained demand. Growth is projected at a CAGR of 6.0%–9.0% through 2030, supported by expanding demographic reach beyond traditional female consumers to include male clientele and younger age cohorts seeking preventative aesthetic interventions. Robust insurance reimbursement for therapeutic indications (chronic migraine, hyperhidrosis, overactive bladder) alongside cash-pay cosmetic volumes create diversified revenue streams. Canada exhibits parallel trends with growing acceptance in urban centers, though price sensitivity and regulatory considerations moderate uptake compared to the U.S. market.
● Europe: The European market demonstrates steady expansion, with projected CAGR spanning 5.0%–8.0% through 2030. Germany, France, the United Kingdom, and Italy represent core markets characterized by sophisticated aesthetic medicine practices, regulatory frameworks emphasizing safety and efficacy evidence, and increasing consumer willingness to pursue non-invasive rejuvenation. The region benefits from established dermatology and plastic surgery training infrastructures that promote best-practice injection techniques. Eastern European markets including Poland and Czech Republic show accelerating adoption as economic development supports discretionary aesthetic spending, though price-conscious consumers favor value-oriented treatment options.
● Asia Pacific: This region emerges as the most dynamic growth frontier, with CAGR estimates ranging from 7.0%–10.0% through 2030, reflecting rapid economic development, expanding middle-class populations, and evolving beauty standards emphasizing youthful appearance. China drives regional momentum through urbanization, rising disposable incomes, and social media influence amplifying aesthetic awareness, with domestic manufacturers increasingly capturing market share through competitive pricing. Japan and South Korea represent mature, sophisticated markets with exceptionally high per-capita aesthetic procedure rates, supported by cultural emphasis on appearance and comprehensive medical aesthetics infrastructure. India presents substantial growth potential as metropolitan areas develop aesthetic medicine capabilities, though affordability constraints and regulatory complexities moderate near-term expansion. Southeast Asian markets including Thailand, Philippines, and Vietnam benefit from medical tourism and improving access to qualified practitioners.
● Latin America: The region exhibits promising growth trajectories, with CAGR projections of 6.0%–9.0% through 2030. Brazil anchors regional dynamics as a global leader in cosmetic procedures, with well-established plastic surgery traditions and broad acceptance of aesthetic interventions across socioeconomic segments. Mexico follows with expanding private aesthetic clinic networks serving domestic and U.S. medical tourism demand. However, economic volatility, currency fluctuations, and import dependency for products create market uncertainties. Affordability remains a key consideration, with growing interest in value-conscious treatment alternatives.
● Middle East and Africa: This region represents an emerging frontier with growth projected at 5.5%–8.5% CAGR through 2030. The United Arab Emirates and Saudi Arabia lead through healthcare infrastructure investments, high-net-worth populations, and cultural shifts toward aesthetic procedures, particularly in expatriate communities. South Africa advances aesthetic medicine capabilities in urban centers, though economic constraints and limited insurance coverage restrict broader population access. Most African markets remain nascent due to affordability barriers, limited trained practitioner networks, and infrastructure gaps, though urban elite segments demonstrate growing interest.
● Company Profiles
● AbbVie: The undisputed global market leader, AbbVie dominates through its Botox Cosmetic franchise, achieving revenues between USD 2.5–3 billion in 2024 specifically from cosmetic botulinum toxin injections. This performance excludes substantial additional revenues from Botox therapeutic indications including chronic migraine, hyperhidrosis, and overactive bladder. AbbVie's market leadership stems from Botox's first-mover advantage, extensive clinical validation across multiple indications, robust FDA and international regulatory approvals, and unparalleled brand recognition that has achieved near-generic status in consumer awareness. The company invests heavily in clinical research expanding indications, practitioner education programs, and consumer marketing emphasizing safety and efficacy. AbbVie's vertically integrated manufacturing, global distribution infrastructure, and pharmaceutical-grade quality systems create formidable competitive barriers.
● Galderma: The second-largest global player, Galderma generated combined revenues of USD 1–1.5 billion in 2024 from its botulinum toxin portfolio comprising Relifyss™, Alluzience®, and Dysport®. Galderma positions itself through multiple formulations addressing diverse practitioner preferences and patient needs, with Dysport providing competitive differentiation through different diffusion characteristics suitable for larger treatment areas. The company leverages its broader aesthetic dermatology portfolio, including dermal fillers and skincare products, to offer comprehensive aesthetic solutions through dermatology and plastic surgery channels. Galderma's strategic focus on practitioner education, clinical evidence generation, and premium positioning supports sustained market presence despite intense competition.
● Merz Pharma GmbH & Co. KGaA: A significant European player, Merz competes through its Xeomin® (incobotulinumtoxinA) formulation distinguished as a "naked" botulinum toxin free of complexing proteins, theoretically reducing immunogenicity risk and antibody formation. Merz targets both aesthetic and therapeutic markets, with particular strength in European markets where its German heritage and clinical focus resonate with practitioners emphasizing evidence-based medicine. The company's integrated approach combining botulinum toxins with hyaluronic acid fillers and skincare positions it as a comprehensive aesthetic solutions provider.
● Revance Therapeutics Inc.: An innovative challenger, Revance developed Daxxify® (daxibotulinumtoxinA), featuring a novel peptide formulation designed to extend duration of effect beyond traditional three-to-four-month injection intervals. This differentiation addresses a key patient and practitioner pain point—treatment frequency—potentially reducing annual visit requirements and improving convenience. Revance targets U.S. aesthetic markets through differentiated positioning, though market adoption requires overcoming incumbent preference for established products and demonstrating consistent long-duration performance across diverse patient populations.
● Hugel Inc., Ipsen Biopharmaceuticals Inc., Evolus Inc.: These companies represent competitive challengers offering biosimilar or competitive formulations targeting price-conscious segments and expanding global access. Hugel's Botulax®, Ipsen's Dysport® (in partnership with Galderma in certain regions), and Evolus's Jeuveau® (daxibotulinumtoxinA-lanm) pursue market share through competitive pricing, targeted geographic expansion, and differentiated positioning emphasizing specific use cases or patient populations. Their strategies capitalize on patent expirations, regulatory pathways for biosimilar approval, and growing practitioner willingness to evaluate alternatives to market leaders based on clinical evidence and cost considerations.
● Asian Manufacturers: Companies including Lanzhou Institute of Biological Products Co. Ltd., JETEMA Co. Ltd., Daewoong Pharmaceutical, Huons BioPharma, Medytox, and ATGC Co. Ltd. represent a growing contingent of Asian manufacturers primarily serving domestic markets with cost-competitive formulations. These players leverage regional manufacturing advantages, government support for biopharmaceutical development, and intimate understanding of local market dynamics to capture share in high-growth Asian markets. Several pursue international expansion through regulatory submissions in Western markets and strategic partnerships, though face challenges related to brand recognition, clinical validation requirements, and established competitor relationships.
● Chongqing Yuyan Pharmaceutical Co. Ltd.: As mentioned, this company represents a potential disruptor through YY001, the world's first recombinant Type A botulinum toxin to reach regulatory submission stage. If approved, YY001 could establish new safety and consistency benchmarks, potentially accelerating industry transition toward recombinant manufacturing and challenging natural formulation incumbents. Success would position Chongqing Yuyan as a technology leader and create opportunities for licensing, partnerships, and global expansion based on superior manufacturing capabilities.
Industry Value Chain Analysis
● The botulinum toxin injection value chain commences with intensive research and development activities spanning bacterial strain selection or genetic engineering for recombinant variants, fermentation process optimization, purification methodology refinement, and formulation development to achieve stability, potency consistency, and safety profiles meeting stringent regulatory standards. R&D demands substantial capital investment over multi-year timelines, incorporating preclinical toxicology studies, mechanism-of-action validation, and extensive clinical trials demonstrating safety and efficacy across targeted indications. Regulatory strategy encompasses engagement with FDA, EMA, NMPA, and other global authorities to navigate complex approval pathways, particularly for novel recombinant formulations requiring comprehensive biological characterization.
● Manufacturing represents a high-complexity, heavily regulated phase involving bioreactor-based fermentation under strict aseptic conditions, multi-step purification employing chromatography and ultrafiltration to remove bacterial components and achieve pharmaceutical-grade purity, formulation into liquid or lyophilized preparations with excipients ensuring stability and reconstitution properties, and aseptic filling into glass vials under cleanroom environments meeting cGMP standards. Quality assurance incorporates potency testing through animal bioassays or validated in vitro methods, sterility verification, endotoxin quantification, protein characterization, and stability studies under various storage conditions. Supply chain management addresses cold-chain logistics requirements for temperature-sensitive biological products, with traceability systems tracking batch genealogy from raw materials through distribution.
● Distribution channels vary by region and regulatory framework, encompassing direct pharmaceutical distribution to hospitals, clinics, and medical spas; specialty distributors with cold-chain capabilities serving aesthetic medicine practitioners; and pharmacy channels for therapeutic indications with insurance reimbursement. Emerging markets increasingly rely on local distributors with established practitioner networks and regulatory expertise navigating import licensing and product registration requirements. Counterfeit products represent a persistent challenge, necessitating anti-counterfeiting measures including serialization, authentication technologies, and practitioner education to identify legitimate products.
● Marketing and commercialization strategies emphasize multi-channel approaches targeting both practitioners and end consumers. Practitioner-directed efforts include medical education through symposia, workshops, and certification programs teaching injection techniques and anatomical considerations; key opinion leader cultivation through research collaborations and speaking engagements; and peer-reviewed publications demonstrating clinical outcomes. Direct-to-consumer marketing, where permitted by regulatory frameworks, leverages social media, digital advertising, and celebrity endorsements to build brand awareness and drive patient consultations, though faces increasing scrutiny regarding claims substantiation and ethical marketing practices. The rise of patient-reported outcomes, before-after photography portfolios, and online reviews significantly influences treatment decisions, shifting power dynamics toward consumer preferences.
● Post-market activities encompass adverse event monitoring through pharmacovigilance systems tracking injection-related complications including bruising, asymmetry, ptosis, and rare systemic effects; ongoing clinical research expanding indications and generating real-world evidence; and iterative product improvements addressing shelf-life extension, reduced reconstitution complexity, or enhanced delivery devices. Sustainability initiatives gain prominence, addressing single-use syringe waste, packaging materials, and fermentation process environmental footprints, though remain nascent compared to pharmaceutical industry broadly.
Opportunities
● Demographic Expansion: Aging global populations seeking age-appropriate aesthetic maintenance, combined with younger cohorts pursuing preventative treatments in their 20s and 30s, dramatically expand addressable patient populations beyond traditional 40–60-year-old demographics.
● Male Market Growth: Increasing cultural acceptance of aesthetic procedures among men, particularly in professional contexts where youthful appearance correlates with career advancement, opens substantial untapped market segments with distinct treatment patterns and product preferences.
● Geographic Penetration: Emerging markets in Asia Pacific, Latin America, and Middle East offer exceptional growth potential as rising disposable incomes, urbanization, and Western beauty standard adoption drive demand among expanding middle-class populations.
● Indication Expansion: Ongoing clinical research exploring novel aesthetic applications including jawline contouring, neck rejuvenation, and facial shaping, alongside therapeutic indications for conditions like bruxism, temporomandibular joint disorders, and neuropathic pain, continuously expand addressable markets.
● Recombinant Technology Adoption: Successful commercialization of recombinant formulations offering superior safety profiles, reduced immunogenicity, and enhanced batch consistency could drive market share shifts toward innovative manufacturers while elevating overall market standards and consumer confidence.
● Combination Therapies: Integration with complementary aesthetic treatments including dermal fillers, energy-based devices, and skincare regimens creates comprehensive facial rejuvenation protocols, increasing per-patient revenues and treatment frequency through synergistic effects and enhanced outcomes.
● Extended-Duration Formulations: Products demonstrating prolonged efficacy beyond traditional three-to-four-month intervals address key patient convenience preferences and practitioner efficiency goals, potentially commanding premium pricing and driving market growth through improved value propositions.
● Digital Health Integration: Telemedicine consultations, AI-powered facial analysis tools, and digital patient management platforms enhance patient access, treatment planning precision, and follow-up care, reducing barriers to entry and improving treatment experiences.
Challenges
● Regulatory Complexity: Stringent biological product regulations requiring extensive clinical validation, ongoing pharmacovigilance, and periodic reinspection of manufacturing facilities create substantial barriers to entry and compliance costs, particularly for smaller manufacturers and recombinant formulations requiring novel regulatory pathways.
● Safety and Efficacy Concerns: Adverse events related to improper injection techniques, anatomical variations, or product quality inconsistencies can damage brand reputation and trigger regulatory scrutiny. High-profile complications amplified through social media create consumer apprehension and potentially drive regulatory restrictions.
● Practitioner Training Gaps: The proliferation of non-specialist injectors including nurses, physician assistants, and aestheticians with varying training standards raises safety concerns and outcome variability. Inadequate anatomical knowledge increases complication risks, while inexperienced injectors may produce suboptimal aesthetic results that undermine patient satisfaction and market growth.
● Counterfeit Products: Illicit manufacturing and distribution of counterfeit or substandard botulinum toxin formulations, particularly in price-sensitive markets with weak regulatory enforcement, threatens patient safety, damages legitimate manufacturer reputations, and erodes consumer confidence in treatment safety.
● Reimbursement Constraints: Limited insurance coverage for cosmetic indications restricts access to cash-pay patients, while reimbursement challenges for therapeutic indications despite demonstrated efficacy limit market expansion. Prior authorization requirements, coverage denials, and inadequate reimbursement rates discourage practitioner adoption for covered indications.
● Competitive Intensity: Market saturation in developed regions combined with entry of biosimilar and recombinant competitors intensifies price competition, erodes profit margins, and necessitates increased marketing expenditures to maintain market share. Practitioner loyalty to established brands creates hurdles for new entrants despite competitive pricing or superior formulations.
● Consumer Price Sensitivity: Economic downturns, inflation, and discretionary spending reductions disproportionately impact cash-pay aesthetic procedures, creating demand volatility. High per-treatment costs ranging from hundreds to over a thousand dollars create affordability barriers for middle-income consumers, limiting market penetration despite strong aspirational demand.
● Cultural and Ethical Considerations: Societal debates regarding beauty standards, body modification ethics, and aesthetic procedure normalization influence consumer attitudes and regulatory approaches. Concerns about unrealistic beauty expectations, particularly among adolescents exposed to filtered social media imagery, may prompt age restrictions or advertising limitations.
Chapter 1 Executive Summary
Chapter 2 Abbreviation and Acronyms
Chapter 3 Preface
3.1 Research Scope
3.2 Research Sources
3.2.1 Data Sources
3.2.2 Assumptions
3.3 Research Method
Chapter 4 Market Landscape
4.1 Market Overview
4.2 Classification/Types
4.3 Application/End Users
Chapter 5 Market Trend Analysis
5.1 introduction
5.2 Drivers
5.3 Restraints
5.4 Opportunities
5.5 Threats
Chapter 6 industry Chain Analysis
6.1 Upstream/Suppliers Analysis
6.2 Botulinum Toxin Injection Analysis
6.2.1 Technology Analysis
6.2.2 Cost Analysis
6.2.3 Market Channel Analysis
6.3 Downstream Buyers/End Users
Chapter 7 Latest Market Dynamics
7.1 Latest News
7.2 Merger and Acquisition
7.3 Planned/Future Project
7.4 Policy Dynamics
Chapter 8 Trading Analysis
8.1 Export of Botulinum Toxin Injection by Region
8.2 Import of Botulinum Toxin Injection by Region
8.3 Balance of Trade
Chapter 9 Historical and Forecast Botulinum Toxin Injection Market in North America (2020-2030)
9.1 Botulinum Toxin Injection Market Size
9.2 Botulinum Toxin Injection Demand by End Use
9.3 Competition by Players/Suppliers
9.4 Type Segmentation and Price
9.5 Key Countries Analysis
9.5.1 United States
9.5.2 Canada
9.5.3 Mexico
Chapter 10 Historical and Forecast Botulinum Toxin Injection Market in South America (2020-2030)
10.1 Botulinum Toxin Injection Market Size
10.2 Botulinum Toxin Injection Demand by End Use
10.3 Competition by Players/Suppliers
10.4 Type Segmentation and Price
10.5 Key Countries Analysis
10.5.1 Brazil
10.5.2 Argentina
10.5.3 Chile
10.5.4 Peru
Chapter 11 Historical and Forecast Botulinum Toxin Injection Market in Asia & Pacific (2020-2030)
11.1 Botulinum Toxin Injection Market Size
11.2 Botulinum Toxin Injection Demand by End Use
11.3 Competition by Players/Suppliers
11.4 Type Segmentation and Price
11.5 Key Countries Analysis
11.5.1 China
11.5.2 india
11.5.3 Japan
11.5.4 South Korea
11.5.5 Southest Asia
11.5.6 Australia
Chapter 12 Historical and Forecast Botulinum Toxin Injection Market in Europe (2020-2030)
12.1 Botulinum Toxin Injection Market Size
12.2 Botulinum Toxin Injection Demand by End Use
12.3 Competition by Players/Suppliers
12.4 Type Segmentation and Price
12.5 Key Countries Analysis
12.5.1 Germany
12.5.2 France
12.5.3 United Kingdom
12.5.4 Italy
12.5.5 Spain
12.5.6 Belgium
12.5.7 Netherlands
12.5.8 Austria
12.5.9 Poland
12.5.10 Russia
Chapter 13 Historical and Forecast Botulinum Toxin Injection Market in MEA (2020-2030)
13.1 Botulinum Toxin Injection Market Size
13.2 Botulinum Toxin Injection Demand by End Use
13.3 Competition by Players/Suppliers
13.4 Type Segmentation and Price
13.5 Key Countries Analysis
13.5.1 Egypt
13.5.2 Israel
13.5.3 South Africa
13.5.4 Gulf Cooperation Council Countries
13.5.5 Turkey
Chapter 14 Summary For Global Botulinum Toxin Injection Market (2020-2025)
14.1 Botulinum Toxin Injection Market Size
14.2 Botulinum Toxin Injection Demand by End Use
14.3 Competition by Players/Suppliers
14.4 Type Segmentation and Price
Chapter 15 Global Botulinum Toxin Injection Market Forecast (2025-2030)
15.1 Botulinum Toxin Injection Market Size Forecast
15.2 Botulinum Toxin Injection Demand Forecast
15.3 Competition by Players/Suppliers
15.4 Type Segmentation and Price Forecast
Chapter 16 Analysis of Global Key Vendors
15.1 AbbVie
15.1.1 Company Profile
15.1.2 Main Business and Botulinum Toxin Injection Information
15.1.3 SWOT Analysis of AbbVie
15.1.4 AbbVie Botulinum Toxin Injection Sales, Revenue, Price and Gross Margin (2020-2025)
15.2 Galderma
15.2.1 Company Profile
15.2.2 Main Business and Botulinum Toxin Injection Information
15.2.3 SWOT Analysis of Galderma
15.2.4 Galderma Botulinum Toxin Injection Sales, Revenue, Price and Gross Margin (2020-2025)
15.3 Merz Pharma GmbH & Co. KGaA
15.3.1 Company Profile
15.3.2 Main Business and Botulinum Toxin Injection Information
15.3.3 SWOT Analysis of Merz Pharma GmbH & Co. KGaA
15.3.4 Merz Pharma GmbH & Co. KGaA Botulinum Toxin Injection Sales, Revenue, Price and Gross Margin (2020-2025)
15.4 Revance Therapeutics Inc
15.4.1 Company Profile
15.4.2 Main Business and Botulinum Toxin Injection Information
15.4.3 SWOT Analysis of Revance Therapeutics Inc
15.4.4 Revance Therapeutics Inc Botulinum Toxin Injection Sales, Revenue, Price and Gross Margin (2020-2025)
15.5 Hugel Inc.
15.5.1 Company Profile
15.5.2 Main Business and Botulinum Toxin Injection Information
15.5.3 SWOT Analysis of Hugel Inc.
15.5.4 Hugel Inc. Botulinum Toxin Injection Sales, Revenue, Price and Gross Margin (2020-2025)
15.6 Ipsen Biopharmaceuticals Inc.
15.6.1 Company Profile
15.6.2 Main Business and Botulinum Toxin Injection Information
15.6.3 SWOT Analysis of Ipsen Biopharmaceuticals Inc.
15.6.4 Ipsen Biopharmaceuticals Inc. Botulinum Toxin Injection Sales, Revenue, Price and Gross Margin (2020-2025)
15.7 Evolus Inc
15.7.1 Company Profile
15.7.2 Main Business and Botulinum Toxin Injection Information
15.7.3 SWOT Analysis of Evolus Inc
15.7.4 Evolus Inc Botulinum Toxin Injection Sales, Revenue, Price and Gross Margin (2020-2025)
15.8 Lanzhou Institute of Biological Products Co. Ltd.
15.8.1 Company Profile
15.8.2 Main Business and Botulinum Toxin Injection Information
15.8.3 SWOT Analysis of Lanzhou Institute of Biological Products Co. Ltd.
15.8.4 Lanzhou Institute of Biological Products Co. Ltd. Botulinum Toxin Injection Sales, Revenue, Price and Gross Margin (2020-2025)
15.9 JETEMA Co. Ltd.
15.9.1 Company Profile
15.9.2 Main Business and Botulinum Toxin Injection Information
15.9.3 SWOT Analysis of JETEMA Co. Ltd.
15.9.4 JETEMA Co. Ltd. Botulinum Toxin Injection Sales, Revenue, Price and Gross Margin (2020-2025)
15.10 Solstice Neurosciences LLC
15.10.1 Company Profile
15.10.2 Main Business and Botulinum Toxin Injection Information
15.10.3 SWOT Analysis of Solstice Neurosciences LLC
15.10.4 Solstice Neurosciences LLC Botulinum Toxin Injection Sales, Revenue, Price and Gross Margin (2020-2025)
15.11 Daewoong Pharmaceutical
15.11.1 Company Profile
15.11.2 Main Business and Botulinum Toxin Injection Information
15.11.3 SWOT Analysis of Daewoong Pharmaceutical
15.11.4 Daewoong Pharmaceutical Botulinum Toxin Injection Sales, Revenue, Price and Gross Margin (2020-2025)
15.12 Huons BioPharma
15.12.1 Company Profile
15.12.2 Main Business and Botulinum Toxin Injection Information
15.12.3 SWOT Analysis of Huons BioPharma
15.12.4 Huons BioPharma Botulinum Toxin Injection Sales, Revenue, Price and Gross Margin (2020-2025)
15.13 Medytox
15.13.1 Company Profile
15.13.2 Main Business and Botulinum Toxin Injection Information
15.13.3 SWOT Analysis of Medytox
15.13.4 Medytox Botulinum Toxin Injection Sales, Revenue, Price and Gross Margin (2020-2025)
15.14 Chongqing Yuyan Pharmaceutical Co. Ltd
15.14.1 Company Profile
15.14.2 Main Business and Botulinum Toxin Injection Information
15.14.3 SWOT Analysis of Chongqing Yuyan Pharmaceutical Co. Ltd
15.14.4 Chongqing Yuyan Pharmaceutical Co. Ltd Botulinum Toxin Injection Sales, Revenue, Price and Gross Margin (2020-2025)
15.15 ATGC Co. Ltd.
15.15.1 Company Profile
15.15.2 Main Business and Botulinum Toxin Injection Information
15.15.3 SWOT Analysis of ATGC Co. Ltd.
15.15.4 ATGC Co. Ltd. Botulinum Toxin Injection Sales, Revenue, Price and Gross Margin (2020-2025)
Please ask for sample pages for full companies list
Table Research Scope of Botulinum Toxin Injection Report
Table Data Sources of Botulinum Toxin Injection Report
Table Major Assumptions of Botulinum Toxin Injection Report
Table Botulinum Toxin Injection Classification
Table Botulinum Toxin Injection Applications List
Table Drivers of Botulinum Toxin Injection Market
Table Restraints of Botulinum Toxin Injection Market
Table Opportunities of Botulinum Toxin Injection Market
Table Threats of Botulinum Toxin Injection Market
Table Raw Materials Suppliers List
Table Different Production Methods of Botulinum Toxin Injection
Table Cost Structure Analysis of Botulinum Toxin Injection
Table Key End Users List
Table Latest News of Botulinum Toxin Injection Market
Table Merger and Acquisition List
Table Planned/Future Project of Botulinum Toxin Injection Market
Table Policy of Botulinum Toxin Injection Market
Table 2020-2030 Regional Export of Botulinum Toxin Injection
Table 2020-2030 Regional Import of Botulinum Toxin Injection
Table 2020-2030 Regional Trade Balance
Table 2020-2030 North America Botulinum Toxin Injection Market Size and Market Volume List
Table 2020-2030 North America Botulinum Toxin Injection Demand List by Application
Table 2020-2025 North America Botulinum Toxin Injection Key Players Sales List
Table 2020-2025 North America Botulinum Toxin Injection Key Players Market Share List
Table 2020-2030 North America Botulinum Toxin Injection Demand List by Type
Table 2020-2025 North America Botulinum Toxin Injection Price List by Type
Table 2020-2030 United States Botulinum Toxin Injection Market Size and Market Volume List
Table 2020-2030 United States Botulinum Toxin Injection Import & Export List
Table 2020-2030 Canada Botulinum Toxin Injection Market Size and Market Volume List
Table 2020-2030 Canada Botulinum Toxin Injection Import & Export List
Table 2020-2030 Mexico Botulinum Toxin Injection Market Size and Market Volume List
Table 2020-2030 Mexico Botulinum Toxin Injection Import & Export List
Table 2020-2030 South America Botulinum Toxin Injection Market Size and Market Volume List
Table 2020-2030 South America Botulinum Toxin Injection Demand List by Application
Table 2020-2025 South America Botulinum Toxin Injection Key Players Sales List
Table 2020-2025 South America Botulinum Toxin Injection Key Players Market Share List
Table 2020-2030 South America Botulinum Toxin Injection Demand List by Type
Table 2020-2025 South America Botulinum Toxin Injection Price List by Type
Table 2020-2030 Brazil Botulinum Toxin Injection Market Size and Market Volume List
Table 2020-2030 Brazil Botulinum Toxin Injection Import & Export List
Table 2020-2030 Argentina Botulinum Toxin Injection Market Size and Market Volume List
Table 2020-2030 Argentina Botulinum Toxin Injection Import & Export List
Table 2020-2030 Chile Botulinum Toxin Injection Market Size and Market Volume List
Table 2020-2030 Chile Botulinum Toxin Injection Import & Export List
Table 2020-2030 Peru Botulinum Toxin Injection Market Size and Market Volume List
Table 2020-2030 Peru Botulinum Toxin Injection Import & Export List
Table 2020-2030 Asia & Pacific Botulinum Toxin Injection Market Size and Market Volume List
Table 2020-2030 Asia & Pacific Botulinum Toxin Injection Demand List by Application
Table 2020-2025 Asia & Pacific Botulinum Toxin Injection Key Players Sales List
Table 2020-2025 Asia & Pacific Botulinum Toxin Injection Key Players Market Share List
Table 2020-2030 Asia & Pacific Botulinum Toxin Injection Demand List by Type
Table 2020-2025 Asia & Pacific Botulinum Toxin Injection Price List by Type
Table 2020-2030 China Botulinum Toxin Injection Market Size and Market Volume List
Table 2020-2030 China Botulinum Toxin Injection Import & Export List
Table 2020-2030 India Botulinum Toxin Injection Market Size and Market Volume List
Table 2020-2030 India Botulinum Toxin Injection Import & Export List
Table 2020-2030 Japan Botulinum Toxin Injection Market Size and Market Volume List
Table 2020-2030 Japan Botulinum Toxin Injection Import & Export List
Table 2020-2030 South Korea Botulinum Toxin Injection Market Size and Market Volume List
Table 2020-2030 South Korea Botulinum Toxin Injection Import & Export List
Table 2020-2030 Southeast Asia Botulinum Toxin Injection Market Size List
Table 2020-2030 Southeast Asia Botulinum Toxin Injection Market Volume List
Table 2020-2030 Southeast Asia Botulinum Toxin Injection Import List
Table 2020-2030 Southeast Asia Botulinum Toxin Injection Export List
Table 2020-2030 Australia Botulinum Toxin Injection Market Size and Market Volume List
Table 2020-2030 Australia Botulinum Toxin Injection Import & Export List
Table 2020-2030 Europe Botulinum Toxin Injection Market Size and Market Volume List
Table 2020-2030 Europe Botulinum Toxin Injection Demand List by Application
Table 2020-2025 Europe Botulinum Toxin Injection Key Players Sales List
Table 2020-2025 Europe Botulinum Toxin Injection Key Players Market Share List
Table 2020-2030 Europe Botulinum Toxin Injection Demand List by Type
Table 2020-2025 Europe Botulinum Toxin Injection Price List by Type
Table 2020-2030 Germany Botulinum Toxin Injection Market Size and Market Volume List
Table 2020-2030 Germany Botulinum Toxin Injection Import & Export List
Table 2020-2030 France Botulinum Toxin Injection Market Size and Market Volume List
Table 2020-2030 France Botulinum Toxin Injection Import & Export List
Table 2020-2030 United Kingdom Botulinum Toxin Injection Market Size and Market Volume List
Table 2020-2030 United Kingdom Botulinum Toxin Injection Import & Export List
Table 2020-2030 Italy Botulinum Toxin Injection Market Size and Market Volume List
Table 2020-2030 Italy Botulinum Toxin Injection Import & Export List
Table 2020-2030 Spain Botulinum Toxin Injection Market Size and Market Volume List
Table 2020-2030 Spain Botulinum Toxin Injection Import & Export List
Table 2020-2030 Belgium Botulinum Toxin Injection Market Size and Market Volume List
Table 2020-2030 Belgium Botulinum Toxin Injection Import & Export List
Table 2020-2030 Netherlands Botulinum Toxin Injection Market Size and Market Volume List
Table 2020-2030 Netherlands Botulinum Toxin Injection Import & Export List
Table 2020-2030 Austria Botulinum Toxin Injection Market Size and Market Volume List
Table 2020-2030 Austria Botulinum Toxin Injection Import & Export List
Table 2020-2030 Poland Botulinum Toxin Injection Market Size and Market Volume List
Table 2020-2030 Poland Botulinum Toxin Injection Import & Export List
Table 2020-2030 Russia Botulinum Toxin Injection Market Size and Market Volume List
Table 2020-2030 Russia Botulinum Toxin Injection Import & Export List
Table 2020-2030 MEA Botulinum Toxin Injection Market Size and Market Volume List
Table 2020-2030 MEA Botulinum Toxin Injection Demand List by Application
Table 2020-2025 MEA Botulinum Toxin Injection Key Players Sales List
Table 2020-2025 MEA Botulinum Toxin Injection Key Players Market Share List
Table 2020-2030 MEA Botulinum Toxin Injection Demand List by Type
Table 2020-2025 MEA Botulinum Toxin Injection Price List by Type
Table 2020-2030 Egypt Botulinum Toxin Injection Market Size and Market Volume List
Table 2020-2030 Egypt Botulinum Toxin Injection Import & Export List
Table 2020-2030 Israel Botulinum Toxin Injection Market Size and Market Volume List
Table 2020-2030 Israel Botulinum Toxin Injection Import & Export List
Table 2020-2030 South Africa Botulinum Toxin Injection Market Size and Market Volume List
Table 2020-2030 South Africa Botulinum Toxin Injection Import & Export List
Table 2020-2030 Gulf Cooperation Council Countries Botulinum Toxin Injection Market Size and Market Volume List
Table 2020-2030 Gulf Cooperation Council Countries Botulinum Toxin Injection Import & Export List
Table 2020-2030 Turkey Botulinum Toxin Injection Market Size and Market Volume List
Table 2020-2030 Turkey Botulinum Toxin Injection Import & Export List
Table 2020-2025 Global Botulinum Toxin Injection Market Size List by Region
Table 2020-2025 Global Botulinum Toxin Injection Market Size Share List by Region
Table 2020-2025 Global Botulinum Toxin Injection Market Volume List by Region
Table 2020-2025 Global Botulinum Toxin Injection Market Volume Share List by Region
Table 2020-2025 Global Botulinum Toxin Injection Demand List by Application
Table 2020-2025 Global Botulinum Toxin Injection Demand Market Share List by Application
Table 2020-2025 Global Botulinum Toxin Injection Key Vendors Sales List
Table 2020-2025 Global Botulinum Toxin Injection Key Vendors Sales Share List
Table 2020-2025 Global Botulinum Toxin Injection Key Vendors Revenue List
Table 2020-2025 Global Botulinum Toxin Injection Key Vendors Revenue Share List
Table 2020-2025 Global Botulinum Toxin Injection Demand List by Type
Table 2020-2025 Global Botulinum Toxin Injection Demand Market Share List by Type
Table 2020-2025 Regional Botulinum Toxin Injection Price List
Table 2025-2030 Global Botulinum Toxin Injection Market Size List by Region
Table 2025-2030 Global Botulinum Toxin Injection Market Size Share List by Region
Table 2025-2030 Global Botulinum Toxin Injection Market Volume List by Region
Table 2025-2030 Global Botulinum Toxin Injection Market Volume Share List by Region
Table 2025-2030 Global Botulinum Toxin Injection Demand List by Application
Table 2025-2030 Global Botulinum Toxin Injection Demand Market Share List by Application
Table 2025-2030 Global Botulinum Toxin Injection Key Vendors Sales List
Table 2025-2030 Global Botulinum Toxin Injection Key Vendors Sales Share List
Table 2025-2030 Global Botulinum Toxin Injection Key Vendors Revenue List
Table 2025-2030 Global Botulinum Toxin Injection Key Vendors Revenue Share List
Table 2025-2030 Global Botulinum Toxin Injection Demand List by Type
Table 2025-2030 Global Botulinum Toxin Injection Demand Market Share List by Type
Table 2025-2030 Botulinum Toxin Injection Regional Price List
Figure Market Size Estimated Method
Figure Major Forecasting Factors
Figure Botulinum Toxin Injection Picture
Figure 2020-2030 Regional Trade Balance
Figure 2020-2030 North America Botulinum Toxin Injection Market Size and CAGR
Figure 2020-2030 North America Botulinum Toxin Injection Market Volume and CAGR
Figure 2020-2030 South America Botulinum Toxin Injection Market Size and CAGR
Figure 2020-2030 South America Botulinum Toxin Injection Market Volume and CAGR
Figure 2020-2030 Asia & Pacific Botulinum Toxin Injection Market Size and CAGR
Figure 2020-2030 Asia & Pacific Botulinum Toxin Injection Market Volume and CAGR
Figure 2020-2030 Europe Botulinum Toxin Injection Market Size and CAGR
Figure 2020-2030 Europe Botulinum Toxin Injection Market Volume and CAGR
Figure 2020-2030 MEA Botulinum Toxin Injection Market Size and CAGR
Figure 2020-2030 MEA Botulinum Toxin Injection Market Volume and CAGR
Figure 2020-2025 Global Botulinum Toxin Injection Market Volume and Growth Rate
Figure 2020-2025 Global Botulinum Toxin Injection Market Size and Growth Rate
Figure 2025-2030 Global Botulinum Toxin Injection Market Volume and Growth Rate
Figure 2025-2030 Global Botulinum Toxin Injection Market Size and Growth Rate
Research Methodology
- Market Estimated Methodology:
Bottom-up & top-down approach, supply & demand approach are the most important method which is used by HDIN Research to estimate the market size.
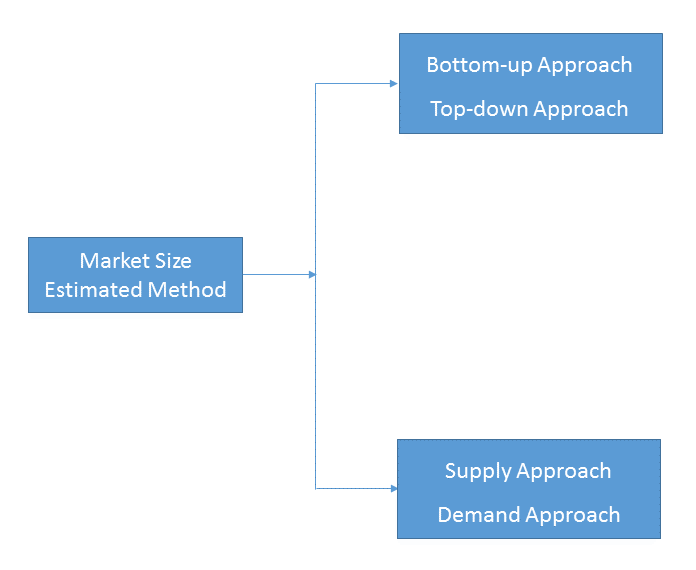
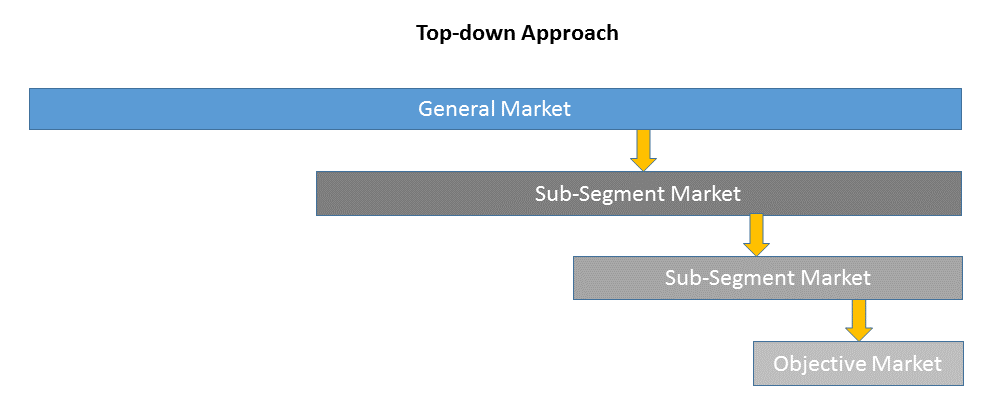
1)Top-down & Bottom-up Approach
Top-down approach uses a general market size figure and determines the percentage that the objective market represents.
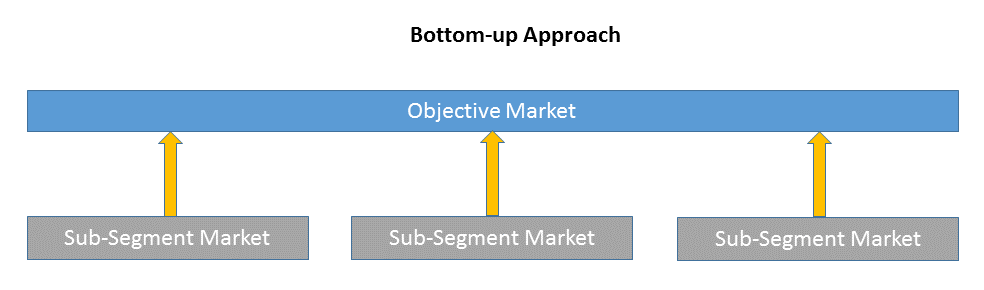
Bottom-up approach size the objective market by collecting the sub-segment information.
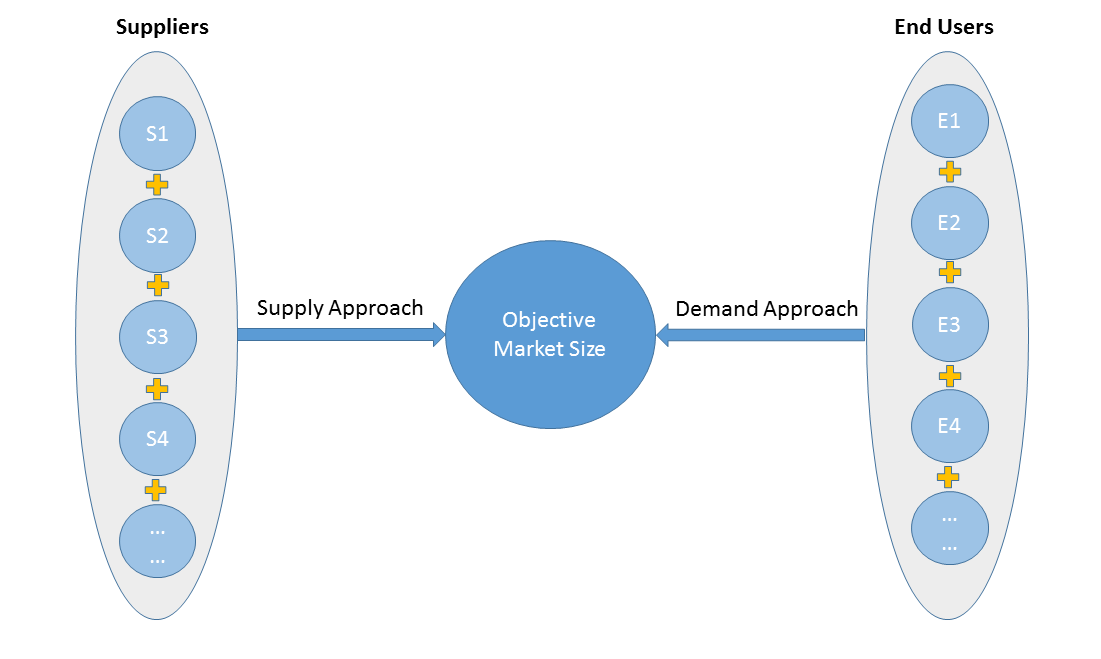
2)Supply & Demand Approach
Supply approach is based on assessments of the size of each competitor supplying the objective market.
Demand approach combine end-user data within a market to estimate the objective market size. It is sometimes referred to as bottom-up approach.
- Forecasting Methodology
- Numerous factors impacting the market trend are considered for forecast model:
- New technology and application in the future;
- New project planned/under contraction;
- Global and regional underlying economic growth;
- Threatens of substitute products;
- Industry expert opinion;
- Policy and Society implication.
- Analysis Tools
1)PEST Analysis
PEST Analysis is a simple and widely used tool that helps our client analyze the Political, Economic, Socio-Cultural, and Technological changes in their business environment.
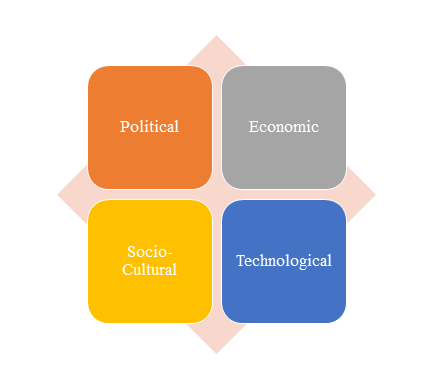
- Benefits of a PEST analysis:
- It helps you to spot business opportunities, and it gives you advanced warning of significant threats.
- It reveals the direction of change within your business environment. This helps you shape what you’re doing, so that you work with change, rather than against it.
- It helps you avoid starting projects that are likely to fail, for reasons beyond your control.
- It can help you break free of unconscious assumptions when you enter a new country, region, or market; because it helps you develop an objective view of this new environment.
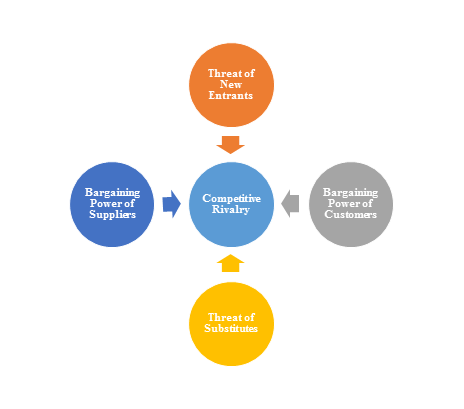
2)Porter’s Five Force Model Analysis
The Porter’s Five Force Model is a tool that can be used to analyze the opportunities and overall competitive advantage. The five forces that can assist in determining the competitive intensity and potential attractiveness within a specific area.
- Threat of New Entrants: Profitable industries that yield high returns will attract new firms.
- Threat of Substitutes: A substitute product uses a different technology to try to solve the same economic need.
- Bargaining Power of Customers: the ability of customers to put the firm under pressure, which also affects the customer's sensitivity to price changes.
- Bargaining Power of Suppliers: Suppliers of raw materials, components, labor, and services (such as expertise) to the firm can be a source of power over the firm when there are few substitutes.
- Competitive Rivalry: For most industries the intensity of competitive rivalry is the major determinant of the competitiveness of the industry.
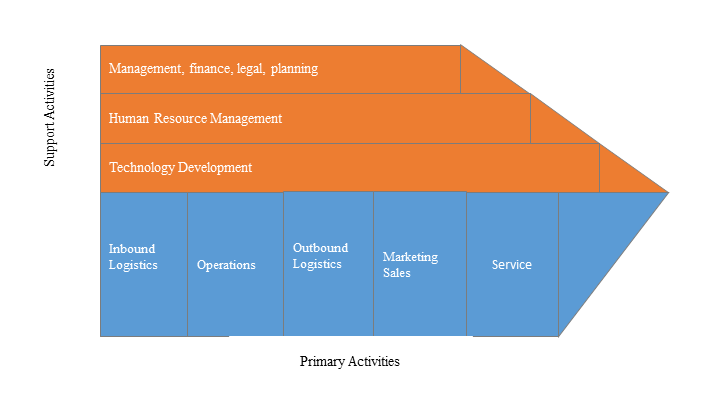
3)Value Chain Analysis
Value chain analysis is a tool to identify activities, within and around the firm and relating these activities to an assessment of competitive strength. Value chain can be analyzed by primary activities and supportive activities. Primary activities include: inbound logistics, operations, outbound logistics, marketing & sales, service. Support activities include: technology development, human resource management, management, finance, legal, planning.
4)SWOT Analysis
SWOT analysis is a tool used to evaluate a company's competitive position by identifying its strengths, weaknesses, opportunities and threats. The strengths and weakness is the inner factor; the opportunities and threats are the external factor. By analyzing the inner and external factors, the analysis can provide the detail information of the position of a player and the characteristics of the industry.
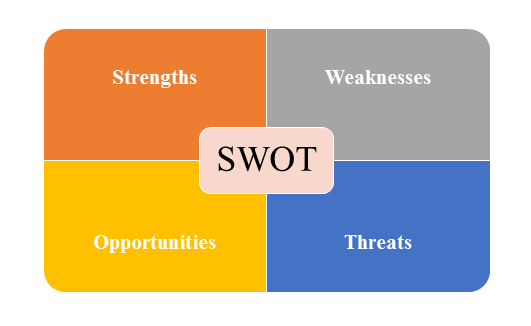
- Strengths describe what the player excels at and separates it from the competition
- Weaknesses stop the player from performing at its optimum level.
- Opportunities refer to favorable external factors that the player can use to give it a competitive advantage.
- Threats refer to factors that have the potential to harm the player.
- Data Sources
| Primary Sources | Secondary Sources |
|---|---|
| Face to face/Phone Interviews with market participants, such as: Manufactures; Distributors; End-users; Experts. Online Survey |
Government/International Organization Data: Annual Report/Presentation/Fact Book Internet Source Information Industry Association Data Free/Purchased Database Market Research Report Book/Journal/News |