T‑cell Therapy Market Insights 2025, Analysis and Forecast to 2030, by Manufacturers, Regions, Technology, Application
- Single User License (1 Users) $ 4,000
- Team License (2~5 Users) $ 5,000
- Corporate License (>5 Users) $ 6,000
Introduction
T-cell therapy represents a revolutionary approach in cancer immunotherapy that harnesses the patient's own immune system to target and destroy malignant cells. This advanced therapeutic modality involves extracting T-cells from patients, genetically modifying or expanding them to recognize specific cancer antigens, and reinfusing them to attack tumor cells. The therapy encompasses multiple technological platforms including CAR-T (Chimeric Antigen Receptor T-cell) therapy, TCR (T-cell Receptor) engineered therapy, and TIL (Tumor-Infiltrating Lymphocyte) therapy. CAR-T technology, discovered in 1986, achieved a landmark milestone in 2017 when Kymriah became the first globally approved CAR-T cell therapy product. The three pioneering companies in CAR-T development—Novartis, Kite Pharma, and Juno Therapeutics—have emerged as global leaders in the cell therapy field. Currently approved CAR-T products primarily target CD19 and BCMA antigens, with marketed indications focused on hematological malignancies. The global market is poised for sustained rapid growth driven by technological breakthroughs in universal CAR-T therapies and cellular treatments for solid tumors, enhanced reimbursement capabilities, frontline treatment advancement, and expanded therapeutic indications.
Market Size and Growth Forecast
The global T-cell therapy market is projected to reach 9.0-11.0 billion USD by 2025, with an estimated compound annual growth rate of 20%-30% through 2030. This exceptional growth trajectory is supported by increasing cancer incidence worldwide, expanding approved indications beyond blood cancers, technological innovations reducing production complexity and costs, growing adoption in earlier treatment lines, and improving reimbursement coverage across major markets. The WHO's International Agency for Research on Cancer projects over 35 million new cancer cases by 2050, representing a 77% increase from the estimated 20 million cases in 2022, creating substantial long-term demand for advanced therapeutic options.
Regional Analysis
North America dominates the T-cell therapy market with estimated growth rates of 22%-32%, driven by the United States which maintains leadership in clinical development, regulatory approvals, and commercial adoption. The region benefits from established biopharmaceutical infrastructure, advanced manufacturing capabilities, comprehensive reimbursement frameworks, and strong academic-industry collaborations supporting innovative therapy development. Major cancer centers and specialized treatment facilities provide extensive patient access to cutting-edge cellular therapies, while robust investment in biotechnology innovation accelerates product development pipelines.
Asia Pacific demonstrates rapid market expansion with growth rates of 18%-28%, led by China which has emerged as a significant hub for CAR-T therapy development and manufacturing. China achieved a notable milestone in November 2024 when Huadao (Shanghai) Biomedical received National Medical Products Administration approval for HD004 CAR-T cell therapy, marking the world's first cellular therapy candidate targeting CLDN18.2-expressing advanced solid tumors with malignant ascites. Japan and South Korea contribute through advanced healthcare systems, growing clinical trial activities, and increasing investment in regenerative medicine. The region benefits from large patient populations, expanding healthcare expenditure, and government initiatives supporting biotechnology development.
Europe exhibits growth rates of 15%-25%, with Germany, the United Kingdom, and France leading in clinical research, regulatory expertise, and specialized treatment infrastructure. The region emphasizes stringent quality standards, comprehensive patient monitoring, and integration of cellular therapies into established oncology treatment paradigms. European markets demonstrate growing acceptance of innovative cancer treatments supported by evolving reimbursement frameworks and expanding treatment center networks.
South America shows emerging growth potential of 12%-20%, with Brazil and Mexico leading due to expanding healthcare infrastructure and growing awareness of advanced cancer treatments. The region faces challenges including limited treatment access, reimbursement constraints, and the need for specialized manufacturing capabilities, but demonstrates increasing interest in cellular therapy adoption.
The Middle East and Africa region demonstrates growth rates of 10%-18%, driven by expanding specialized cancer centers in Gulf states and growing medical tourism for advanced treatments. The region benefits from increasing healthcare investment and partnerships with international biotechnology companies, though widespread adoption remains constrained by infrastructure limitations and cost considerations.
Application Analysis
Liquid Tumor Application: This segment currently dominates the market with projected growth of 18%-28%, encompassing hematological malignancies including B-cell lymphomas, acute lymphoblastic leukemia, and multiple myeloma. All currently approved CAR-T products target blood cancers, with established clinical efficacy and growing real-world evidence supporting expanded use. The segment benefits from well-characterized target antigens, favorable tumor microenvironments for T-cell function, and proven therapeutic responses in heavily pre-treated patients. Key trends include advancement of therapies to earlier treatment lines, development of dual-targeting and multi-specific constructs, and exploration of allogeneic off-the-shelf approaches to address manufacturing complexity and cost constraints.
Solid Tumor Application: Expected to demonstrate the fastest growth at 25%-35%, this segment represents the next frontier in cellular therapy development with significant unmet medical need. Compared to blood cancers, CAR-T application in solid tumors has been limited by challenges including heterogeneous antigen expression, immunosuppressive tumor microenvironments, limited T-cell trafficking to tumor sites, and on-target off-tumor toxicity concerns. The November 2024 approval of HD004 CAR-T therapy targeting CLDN18.2 marks a breakthrough as the world's first approved cellular therapy for solid tumors, specifically addressing advanced solid tumor malignant ascites. This milestone validates technological approaches overcoming solid tumor barriers and opens pathways for expanded indication development. Growth drivers include advancing engineering strategies to enhance T-cell persistence and tumor penetration, combination approaches with checkpoint inhibitors and targeted therapies, and identification of novel tumor-selective antigens enabling safer targeting strategies.
Key Market Players
Novartis: The Swiss pharmaceutical giant maintains pioneering leadership in CAR-T therapy through Kymriah (tisagenlecleucel), the first globally approved CAR-T product targeting CD19-positive hematological malignancies. Novartis operates advanced manufacturing infrastructure and extensive clinical development programs supporting indication expansion and next-generation product development.
Gilead Sciences (Kite Pharma): Through its Kite Pharma subsidiary, Gilead markets Yescarta and Tecartus, important CAR-T therapies for B-cell lymphomas and mantle cell lymphoma. The company maintains strong manufacturing capabilities and active clinical pipelines advancing cellular therapy applications across multiple cancer types.
Bristol Myers Squibb: This American pharmaceutical company acquired Celgene and its Juno Therapeutics subsidiary, gaining access to Breyanzi and Abecma CAR-T therapies. Bristol Myers Squibb leverages extensive oncology expertise and global commercial infrastructure to expand cellular therapy adoption worldwide.
JW Therapeutics: The Chinese biopharmaceutical company focuses on developing and commercializing cellular immunotherapy products for the Chinese market. JW Therapeutics collaborates with international partners while building domestic manufacturing capacity and clinical development capabilities.
Takara Bio: This Japanese biotechnology company specializes in gene and cell therapy technologies, providing manufacturing platforms and developing proprietary cellular therapy products. Takara Bio's technology platforms support both autologous and allogeneic T-cell therapy development.
Iovance Biotherapeutics: This American company leads in tumor-infiltrating lymphocyte therapy development, advancing innovative approaches using naturally occurring tumor-reactive T-cells. Iovance focuses on solid tumor applications with product candidates in advanced clinical development.
BioNTech SE: The German biotechnology company, known for mRNA vaccine technology, actively develops next-generation CAR-T and TCR therapies leveraging proprietary platforms. BioNTech pursues innovative approaches including personalized neoantigen-targeted cellular therapies.
Adaptimmune: This British-American company specializes in engineered TCR therapy development targeting intracellular cancer antigens inaccessible to conventional CAR-T approaches. Adaptimmune advances multiple product candidates for solid tumor applications.
Autolus Therapeutics: The British clinical-stage company develops next-generation programmed CAR-T therapies incorporating proprietary technologies to enhance efficacy and safety. Autolus targets both hematological malignancies and solid tumors through innovative engineering strategies.
Industry Value Chain Analysis
The T-cell therapy industry value chain encompasses sophisticated processes from patient cell collection through complex manufacturing and clinical administration. Upstream activities involve patient leukapheresis procedures extracting peripheral blood mononuclear cells containing T-cells, requiring specialized equipment and trained personnel at qualified collection centers. Collected cells undergo cryopreservation and transportation to manufacturing facilities under controlled conditions maintaining cell viability.
Manufacturing represents the most complex and resource-intensive value chain component, involving multiple sophisticated processes. T-cell isolation and activation utilize immunomagnetic separation technologies and stimulation protocols. Genetic modification employs viral vectors (typically lentiviral or retroviral) or non-viral approaches (electroporation, transposons) to introduce CAR or TCR constructs. Cell expansion requires specialized bioreactors, controlled culture conditions, and extensive quality monitoring to achieve therapeutic doses while maintaining T-cell functionality. Current autologous manufacturing approaches require 2-4 weeks per patient batch, creating logistical complexity and limiting scalability. Emerging allogeneic approaches utilizing donor-derived cells or induced pluripotent stem cells promise off-the-shelf availability but face additional technical challenges including immune rejection prevention.
Quality control and regulatory compliance demand comprehensive analytical testing including identity confirmation, potency assessment, safety evaluation for replication-competent viruses, and sterility verification. Manufacturing facilities require specialized infrastructure including cleanrooms, advanced analytical equipment, and quality management systems meeting regulatory requirements across multiple jurisdictions.
Distribution involves cryopreserved product transportation to treatment centers with sophisticated cold chain logistics ensuring product integrity. Treatment administration requires specialized medical facilities with intensive care capabilities, trained oncology teams experienced in cellular therapy management, and comprehensive patient monitoring protocols for toxicity management including cytokine release syndrome and neurotoxicity.
Clinical support services encompass patient selection and eligibility assessment, bridging therapy management during manufacturing, adverse event monitoring and intervention, and long-term follow-up for efficacy and safety evaluation. The value chain benefits from growing partnerships between biotechnology companies, contract manufacturing organizations, logistics providers, and specialized treatment centers creating integrated service networks.
Market Opportunities and Challenges
Opportunities
●Solid Tumor Expansion: The approval of the first solid tumor-targeted CAR-T therapy validates technological approaches overcoming historical limitations and opens substantial market opportunities. Solid tumors represent significantly larger patient populations than blood cancers, with major cancer types including lung, gastrointestinal, breast, and prostate cancers offering immense therapeutic potential. Successful development of cellular therapies for solid tumors could expand addressable markets exponentially while addressing critical unmet medical needs in high-mortality cancer types.
●Universal Off-the-Shelf Therapies: Development of allogeneic CAR-T and TCR therapies using donor-derived or stem cell-derived T-cells promises to transform market accessibility and economics. Universal approaches eliminate patient-specific manufacturing requirements, reduce treatment timelines from weeks to days, enable inventory management and immediate availability, and substantially lower production costs through economies of scale. Successful allogeneic products could democratize access to cellular therapies across broader patient populations and healthcare systems.
●Earlier Treatment Lines: Advancing cellular therapies from late-line heavily pre-treated settings to earlier treatment positions increases eligible patient populations and potentially improves therapeutic outcomes. Clinical trials demonstrating superiority in earlier lines versus standard chemotherapy or targeted therapy support indication expansion and market growth. Earlier adoption also addresses patients with better performance status and less treatment-resistant disease, potentially enhancing response rates and durability.
●Combination Therapy Strategies: Integration of cellular therapies with complementary treatment modalities including checkpoint inhibitors, targeted agents, chemotherapy, and radiation therapy offers synergistic potential. Combination approaches may overcome resistance mechanisms, enhance T-cell function, and extend response durability. Strategic combinations could expand treatable patient populations and improve outcomes across diverse cancer types.
Reimbursement Evolution: Improving payer coverage and innovative reimbursement models including outcomes-based agreements and installment payment structures address cost barriers limiting adoption. As real-world evidence demonstrates long-term efficacy and potential curative benefits, reimbursement expansion supports market penetration across healthcare systems and geographies.
Challenges
●Manufacturing Complexity and Cost: Current autologous manufacturing processes require sophisticated infrastructure, specialized expertise, and patient-specific production creating significant cost burdens. Manufacturing complexity limits production capacity, constrains patient access, and results in premium pricing that challenges healthcare system affordability. Price constraints and production cycle limitations restrict technology scalability and widespread adoption. Addressing manufacturing challenges requires substantial investment in automation technologies, process optimization, and novel manufacturing platforms.
●Solid Tumor Biology Barriers: Despite recent breakthroughs, cellular therapy application in solid tumors faces substantial biological challenges including tumor antigen heterogeneity limiting complete responses, immunosuppressive microenvironments inhibiting T-cell function, physical barriers preventing T-cell infiltration, and limited tumor-specific antigens creating on-target off-tumor toxicity risks. Overcoming these barriers demands continued innovation in T-cell engineering, combination strategies, and target selection.
●Safety Management: Cellular therapy administration carries risks of serious adverse events including cytokine release syndrome requiring intensive supportive care and neurotoxicity demanding specialized monitoring and intervention. Safety concerns necessitate treatment at specialized centers with experienced teams and intensive care capabilities, limiting treatment accessibility and adding cost. Long-term safety surveillance requirements for genetically modified cell products add complexity to clinical development and post-marketing monitoring.
●Regulatory Complexity: Cellular therapy products face intricate regulatory pathways across global markets with varying requirements for manufacturing standards, analytical methods, clinical evidence, and post-marketing surveillance. Harmonization challenges across jurisdictions complicate global development strategies and market access. Evolving regulatory frameworks for novel approaches including allogeneic products, gene editing technologies, and combination therapies create uncertainty requiring ongoing dialogue with regulatory authorities.
●Trump Administration Tariff Policy Impact: The Trump administration's announcement of 100% tariffs on branded or patented pharmaceutical products imported to the United States, effective October 1, 2025, unless companies establish U.S. manufacturing facilities, creates significant uncertainty for the global T-cell therapy industry. This policy potentially disrupts international supply chains and manufacturing strategies, particularly impacting companies with production facilities outside the United States. The sophisticated infrastructure requirements, regulatory considerations, and substantial capital investment needed for cellular therapy manufacturing facilities complicate rapid U.S. manufacturing establishment. Companies may face difficult choices between absorbing significant tariff costs, passing pricing increases to healthcare systems already challenged by cellular therapy costs, or making substantial investments in domestic manufacturing capacity. The policy could accelerate manufacturing localization trends but may also delay product launches, restrict patient access, and create market fragmentation as companies navigate divergent global manufacturing and supply chain strategies. The uncertainty surrounding tariff implementation and potential exemptions for critical therapeutics adds complexity to strategic planning and investment decisions across the industry.
Chapter 1 Executive Summary
Chapter 2 Abbreviation and Acronyms
Chapter 3 Preface
3.1 Research Scope
3.2 Research Sources
3.2.1 Data Sources
3.2.2 Assumptions
3.3 Research Method
Chapter 4 Market Landscape
4.1 Market Overview
4.2 Classification/Types
4.3 Application/End Users
Chapter 5 Market Trend Analysis
5.1 introduction
5.2 Drivers
5.3 Restraints
5.4 Opportunities
5.5 Threats
Chapter 6 industry Chain Analysis
6.1 Upstream/Suppliers Analysis
6.2 T‑cell Therapy Analysis
6.2.1 Technology Analysis
6.2.2 Cost Analysis
6.2.3 Market Channel Analysis
6.3 Downstream Buyers/End Users
Chapter 7 Latest Market Dynamics
7.1 Latest News
7.2 Merger and Acquisition
7.3 Planned/Future Project
7.4 Policy Dynamics
Chapter 8 Historical and Forecast T‑cell Therapy Market in North America (2020-2030)
8.1 T‑cell Therapy Market Size
8.2 T‑cell Therapy Market by End Use
8.3 Competition by Players/Suppliers
8.4 T‑cell Therapy Market Size by Type
8.5 Key Countries Analysis
8.5.1 United States
8.5.2 Canada
8.5.3 Mexico
Chapter 9 Historical and Forecast T‑cell Therapy Market in South America (2020-2030)
9.1 T‑cell Therapy Market Size
9.2 T‑cell Therapy Market by End Use
9.3 Competition by Players/Suppliers
9.4 T‑cell Therapy Market Size by Type
9.5 Key Countries Analysis
9.5.1 Brazil
9.5.2 Argentina
9.5.3 Chile
9.5.4 Peru
Chapter 10 Historical and Forecast T‑cell Therapy Market in Asia & Pacific (2020-2030)
10.1 T‑cell Therapy Market Size
10.2 T‑cell Therapy Market by End Use
10.3 Competition by Players/Suppliers
10.4 T‑cell Therapy Market Size by Type
10.5 Key Countries Analysis
10.5.1 China
10.5.2 India
10.5.3 Japan
10.5.4 South Korea
10.5.5 Southest Asia
10.5.6 Australia
Chapter 11 Historical and Forecast T‑cell Therapy Market in Europe (2020-2030)
11.1 T‑cell Therapy Market Size
11.2 T‑cell Therapy Market by End Use
11.3 Competition by Players/Suppliers
11.4 T‑cell Therapy Market Size by Type
11.5 Key Countries Analysis
11.5.1 Germany
11.5.2 France
11.5.3 United Kingdom
11.5.4 Italy
11.5.5 Spain
11.5.6 Belgium
11.5.7 Netherlands
11.5.8 Austria
11.5.9 Poland
11.5.10 Russia
Chapter 12 Historical and Forecast T‑cell Therapy Market in MEA (2020-2030)
12.1 T‑cell Therapy Market Size
12.2 T‑cell Therapy Market by End Use
12.3 Competition by Players/Suppliers
12.4 T‑cell Therapy Market Size by Type
12.5 Key Countries Analysis
12.5.1 Egypt
12.5.2 Israel
12.5.3 South Africa
12.5.4 Gulf Cooperation Council Countries
12.5.5 Turkey
Chapter 13 Summary For Global T‑cell Therapy Market (2020-2025)
13.1 T‑cell Therapy Market Size
13.2 T‑cell Therapy Market by End Use
13.3 Competition by Players/Suppliers
13.4 T‑cell Therapy Market Size by Type
Chapter 14 Global T‑cell Therapy Market Forecast (2025-2030)
14.1 T‑cell Therapy Market Size Forecast
14.2 T‑cell Therapy Application Forecast
14.3 Competition by Players/Suppliers
14.4 T‑cell Therapy Type Forecast
Chapter 15 Analysis of Global Key Vendors
15.1 Adaptimmune Ltd.
15.1.1 Company Profile
15.1.2 Main Business and T‑cell Therapy Information
15.1.3 SWOT Analysis of Adaptimmune Ltd.
15.1.4 Adaptimmune Ltd. T‑cell Therapy Sales, Revenue, Price and Gross Margin (2020-2025)
15.2 Adicet Bio
15.2.1 Company Profile
15.2.2 Main Business and T‑cell Therapy Information
15.2.3 SWOT Analysis of Adicet Bio
15.2.4 Adicet Bio T‑cell Therapy Sales, Revenue, Price and Gross Margin (2020-2025)
15.3 Autolus Therapeutics
15.3.1 Company Profile
15.3.2 Main Business and T‑cell Therapy Information
15.3.3 SWOT Analysis of Autolus Therapeutics
15.3.4 Autolus Therapeutics T‑cell Therapy Sales, Revenue, Price and Gross Margin (2020-2025)
15.4 Cell Medica Limited
15.4.1 Company Profile
15.4.2 Main Business and T‑cell Therapy Information
15.4.3 SWOT Analysis of Cell Medica Limited
15.4.4 Cell Medica Limited T‑cell Therapy Sales, Revenue, Price and Gross Margin (2020-2025)
15.5 GlaxoSmithKline
15.5.1 Company Profile
15.5.2 Main Business and T‑cell Therapy Information
15.5.3 SWOT Analysis of GlaxoSmithKline
15.5.4 GlaxoSmithKline T‑cell Therapy Sales, Revenue, Price and Gross Margin (2020-2025)
15.6 Green Cross LabCell Corp.
15.6.1 Company Profile
15.6.2 Main Business and T‑cell Therapy Information
15.6.3 SWOT Analysis of Green Cross LabCell Corp.
15.6.4 Green Cross LabCell Corp. T‑cell Therapy Sales, Revenue, Price and Gross Margin (2020-2025)
15.7 Immunocore Limited
15.7.1 Company Profile
15.7.2 Main Business and T‑cell Therapy Information
15.7.3 SWOT Analysis of Immunocore Limited
15.7.4 Immunocore Limited T‑cell Therapy Sales, Revenue, Price and Gross Margin (2020-2025)
15.8 Iovance Biotherapeutics Inc.
15.8.1 Company Profile
15.8.2 Main Business and T‑cell Therapy Information
15.8.3 SWOT Analysis of Iovance Biotherapeutics Inc.
15.8.4 Iovance Biotherapeutics Inc. T‑cell Therapy Sales, Revenue, Price and Gross Margin (2020-2025)
15.9 Kiadis Pharma Netherlands B.V.
15.9.1 Company Profile
15.9.2 Main Business and T‑cell Therapy Information
15.9.3 SWOT Analysis of Kiadis Pharma Netherlands B.V.
15.9.4 Kiadis Pharma Netherlands B.V. T‑cell Therapy Sales, Revenue, Price and Gross Margin (2020-2025)
15.10 Lion TCR Pte Ltd.
15.10.1 Company Profile
15.10.2 Main Business and T‑cell Therapy Information
15.10.3 SWOT Analysis of Lion TCR Pte Ltd.
15.10.4 Lion TCR Pte Ltd. T‑cell Therapy Sales, Revenue, Price and Gross Margin (2020-2025)
15.11 MolMed
15.11.1 Company Profile
15.11.2 Main Business and T‑cell Therapy Information
15.11.3 SWOT Analysis of MolMed
15.11.4 MolMed T‑cell Therapy Sales, Revenue, Price and Gross Margin (2020-2025)
15.12 Precision Biosciences
15.12.1 Company Profile
15.12.2 Main Business and T‑cell Therapy Information
15.12.3 SWOT Analysis of Precision Biosciences
15.12.4 Precision Biosciences T‑cell Therapy Sales, Revenue, Price and Gross Margin (2020-2025)
15.13 Janssen Pharmaceuticals
15.13.1 Company Profile
15.13.2 Main Business and T‑cell Therapy Information
15.13.3 SWOT Analysis of Janssen Pharmaceuticals
15.13.4 Janssen Pharmaceuticals T‑cell Therapy Sales, Revenue, Price and Gross Margin (2020-2025)
15.14 Noile‑Immune Biotech
15.14.1 Company Profile
15.14.2 Main Business and T‑cell Therapy Information
15.14.3 SWOT Analysis of Noile‑Immune Biotech
15.14.4 Noile‑Immune Biotech T‑cell Therapy Sales, Revenue, Price and Gross Margin (2020-2025)
15.15 Anixa Biosciences
15.15.1 Company Profile
15.15.2 Main Business and T‑cell Therapy Information
15.15.3 SWOT Analysis of Anixa Biosciences
15.15.4 Anixa Biosciences T‑cell Therapy Sales, Revenue, Price and Gross Margin (2020-2025)
15.16 Beam Therapeutics Inc.
15.16.1 Company Profile
15.16.2 Main Business and T‑cell Therapy Information
15.16.3 SWOT Analysis of Beam Therapeutics Inc.
15.16.4 Beam Therapeutics Inc. T‑cell Therapy Sales, Revenue, Price and Gross Margin (2020-2025)
15.17 BioNTech SE
15.17.1 Company Profile
15.17.2 Main Business and T‑cell Therapy Information
15.17.3 SWOT Analysis of BioNTech SE
15.17.4 BioNTech SE T‑cell Therapy Sales, Revenue, Price and Gross Margin (2020-2025)
15.18 Cartesian Therapeutics
15.18.1 Company Profile
15.18.2 Main Business and T‑cell Therapy Information
15.18.3 SWOT Analysis of Cartesian Therapeutics
15.18.4 Cartesian Therapeutics T‑cell Therapy Sales, Revenue, Price and Gross Margin (2020-2025)
15.19 Takara Bio
15.19.1 Company Profile
15.19.2 Main Business and T‑cell Therapy Information
15.19.3 SWOT Analysis of Takara Bio
15.19.4 Takara Bio T‑cell Therapy Sales, Revenue, Price and Gross Margin (2020-2025)
15.20 Nantkwest
15.20.1 Company Profile
15.20.2 Main Business and T‑cell Therapy Information
15.20.3 SWOT Analysis of Nantkwest
15.20.4 Nantkwest T‑cell Therapy Sales, Revenue, Price and Gross Margin (2020-2025)
15.21 Novartis
15.21.1 Company Profile
15.21.2 Main Business and T‑cell Therapy Information
15.21.3 SWOT Analysis of Novartis
15.21.4 Novartis T‑cell Therapy Sales, Revenue, Price and Gross Margin (2020-2025)
15.22 Gilead
15.22.1 Company Profile
15.22.2 Main Business and T‑cell Therapy Information
15.22.3 SWOT Analysis of Gilead
15.22.4 Gilead T‑cell Therapy Sales, Revenue, Price and Gross Margin (2020-2025)
15.23 Bristol Myers Squibb
15.23.1 Company Profile
15.23.2 Main Business and T‑cell Therapy Information
15.23.3 SWOT Analysis of Bristol Myers Squibb
15.23.4 Bristol Myers Squibb T‑cell Therapy Sales, Revenue, Price and Gross Margin (2020-2025)
15.24 JW Therapeutics
15.24.1 Company Profile
15.24.2 Main Business and T‑cell Therapy Information
15.24.3 SWOT Analysis of JW Therapeutics
15.24.4 JW Therapeutics T‑cell Therapy Sales, Revenue, Price and Gross Margin (2020-2025)
Please ask for sample pages for full companies list
Table Research Scope of T‑cell Therapy Report
Table Data Sources of T‑cell Therapy Report
Table Major Assumptions of T‑cell Therapy Report
Table T‑cell Therapy Classification
Table T‑cell Therapy Applications
Table Drivers of T‑cell Therapy Market
Table Restraints of T‑cell Therapy Market
Table Opportunities of T‑cell Therapy Market
Table Threats of T‑cell Therapy Market
Table Raw Materials Suppliers
Table Different Production Methods of T‑cell Therapy
Table Cost Structure Analysis of T‑cell Therapy
Table Key End Users
Table Latest News of T‑cell Therapy Market
Table Merger and Acquisition
Table Planned/Future Project of T‑cell Therapy Market
Table Policy of T‑cell Therapy Market
Table 2020-2030 North America T‑cell Therapy Market Size
Table 2020-2030 North America T‑cell Therapy Market Size by Application
Table 2020-2025 North America T‑cell Therapy Key Players Revenue
Table 2020-2025 North America T‑cell Therapy Key Players Market Share
Table 2020-2030 North America T‑cell Therapy Market Size by Type
Table 2020-2030 United States T‑cell Therapy Market Size
Table 2020-2030 Canada T‑cell Therapy Market Size
Table 2020-2030 Mexico T‑cell Therapy Market Size
Table 2020-2030 South America T‑cell Therapy Market Size
Table 2020-2030 South America T‑cell Therapy Market Size by Application
Table 2020-2025 South America T‑cell Therapy Key Players Revenue
Table 2020-2025 South America T‑cell Therapy Key Players Market Share
Table 2020-2030 South America T‑cell Therapy Market Size by Type
Table 2020-2030 Brazil T‑cell Therapy Market Size
Table 2020-2030 Argentina T‑cell Therapy Market Size
Table 2020-2030 Chile T‑cell Therapy Market Size
Table 2020-2030 Peru T‑cell Therapy Market Size
Table 2020-2030 Asia & Pacific T‑cell Therapy Market Size
Table 2020-2030 Asia & Pacific T‑cell Therapy Market Size by Application
Table 2020-2025 Asia & Pacific T‑cell Therapy Key Players Revenue
Table 2020-2025 Asia & Pacific T‑cell Therapy Key Players Market Share
Table 2020-2030 Asia & Pacific T‑cell Therapy Market Size by Type
Table 2020-2030 China T‑cell Therapy Market Size
Table 2020-2030 India T‑cell Therapy Market Size
Table 2020-2030 Japan T‑cell Therapy Market Size
Table 2020-2030 South Korea T‑cell Therapy Market Size
Table 2020-2030 Southeast Asia T‑cell Therapy Market Size
Table 2020-2030 Australia T‑cell Therapy Market Size
Table 2020-2030 Europe T‑cell Therapy Market Size
Table 2020-2030 Europe T‑cell Therapy Market Size by Application
Table 2020-2025 Europe T‑cell Therapy Key Players Revenue
Table 2020-2025 Europe T‑cell Therapy Key Players Market Share
Table 2020-2030 Europe T‑cell Therapy Market Size by Type
Table 2020-2030 Germany T‑cell Therapy Market Size
Table 2020-2030 France T‑cell Therapy Market Size
Table 2020-2030 United Kingdom T‑cell Therapy Market Size
Table 2020-2030 Italy T‑cell Therapy Market Size
Table 2020-2030 Spain T‑cell Therapy Market Size
Table 2020-2030 Belgium T‑cell Therapy Market Size
Table 2020-2030 Netherlands T‑cell Therapy Market Size
Table 2020-2030 Austria T‑cell Therapy Market Size
Table 2020-2030 Poland T‑cell Therapy Market Size
Table 2020-2030 Russia T‑cell Therapy Market Size
Table 2020-2030 MEA T‑cell Therapy Market Size
Table 2020-2030 MEA T‑cell Therapy Market Size by Application
Table 2020-2025 MEA T‑cell Therapy Key Players Revenue
Table 2020-2025 MEA T‑cell Therapy Key Players Market Share
Table 2020-2030 MEA T‑cell Therapy Market Size by Type
Table 2020-2030 Egypt T‑cell Therapy Market Size
Table 2020-2030 Israel T‑cell Therapy Market Size
Table 2020-2030 South Africa T‑cell Therapy Market Size
Table 2020-2030 Gulf Cooperation Council Countries T‑cell Therapy Market Size
Table 2020-2030 Turkey T‑cell Therapy Market Size
Table 2020-2025 Global T‑cell Therapy Market Size by Region
Table 2020-2025 Global T‑cell Therapy Market Size Share by Region
Table 2020-2025 Global T‑cell Therapy Market Size by Application
Table 2020-2025 Global T‑cell Therapy Market Share by Application
Table 2020-2025 Global T‑cell Therapy Key Vendors Revenue
Table 2020-2025 Global T‑cell Therapy Key Vendors Market Share
Table 2020-2025 Global T‑cell Therapy Market Size by Type
Table 2020-2025 Global T‑cell Therapy Market Share by Type
Table 2025-2030 Global T‑cell Therapy Market Size by Region
Table 2025-2030 Global T‑cell Therapy Market Size Share by Region
Table 2025-2030 Global T‑cell Therapy Market Size by Application
Table 2025-2030 Global T‑cell Therapy Market Share by Application
Table 2025-2030 Global T‑cell Therapy Key Vendors Revenue
Table 2025-2030 Global T‑cell Therapy Key Vendors Market Share
Table 2025-2030 Global T‑cell Therapy Market Size by Type
Table 2025-2030 T‑cell Therapy Global Market Share by Type
Figure Market Size Estimated Method
Figure Major Forecasting Factors
Figure T‑cell Therapy Picture
Figure 2020-2030 North America T‑cell Therapy Market Size and CAGR
Figure 2020-2030 South America T‑cell Therapy Market Size and CAGR
Figure 2020-2030 Asia & Pacific T‑cell Therapy Market Size and CAGR
Figure 2020-2030 Europe T‑cell Therapy Market Size and CAGR
Figure 2020-2030 MEA T‑cell Therapy Market Size and CAGR
Figure 2020-2025 Global T‑cell Therapy Market Size and Growth Rate
Figure 2025-2030 Global T‑cell Therapy Market Size and Growth Rate
Research Methodology
- Market Estimated Methodology:
Bottom-up & top-down approach, supply & demand approach are the most important method which is used by HDIN Research to estimate the market size.
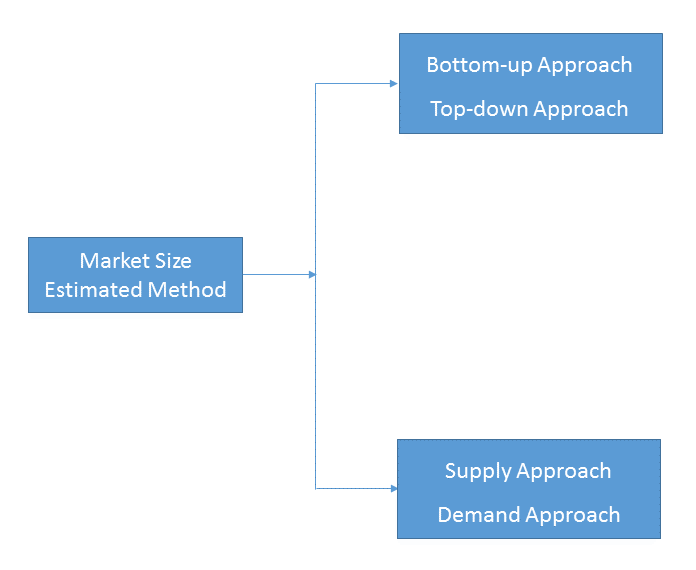
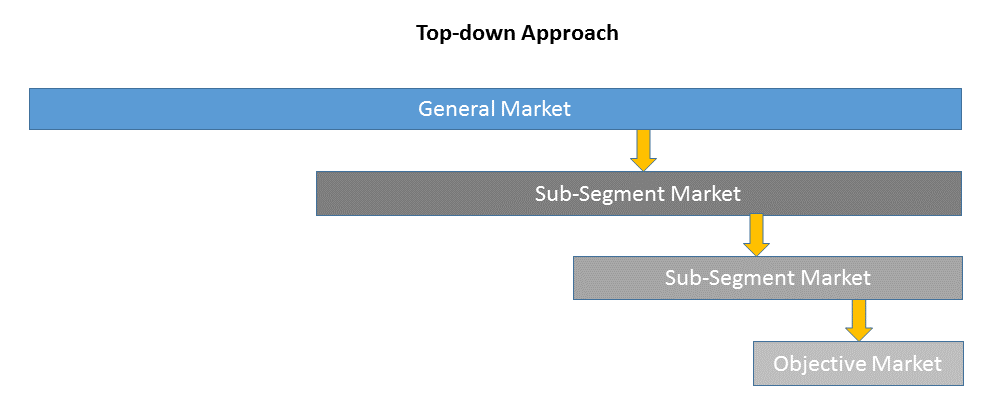
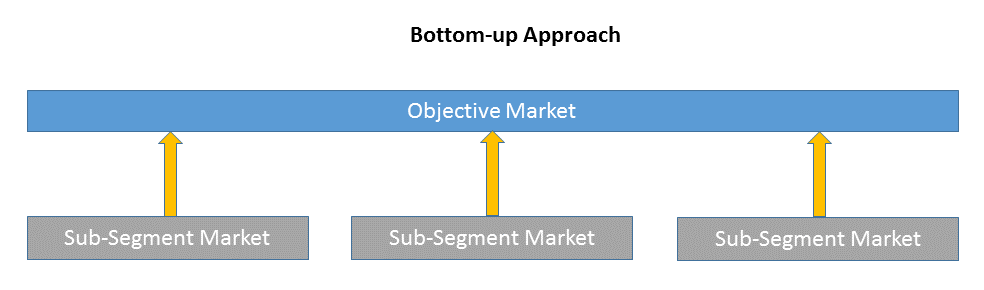
1)Top-down & Bottom-up Approach
Top-down approach uses a general market size figure and determines the percentage that the objective market represents.
Bottom-up approach size the objective market by collecting the sub-segment information.
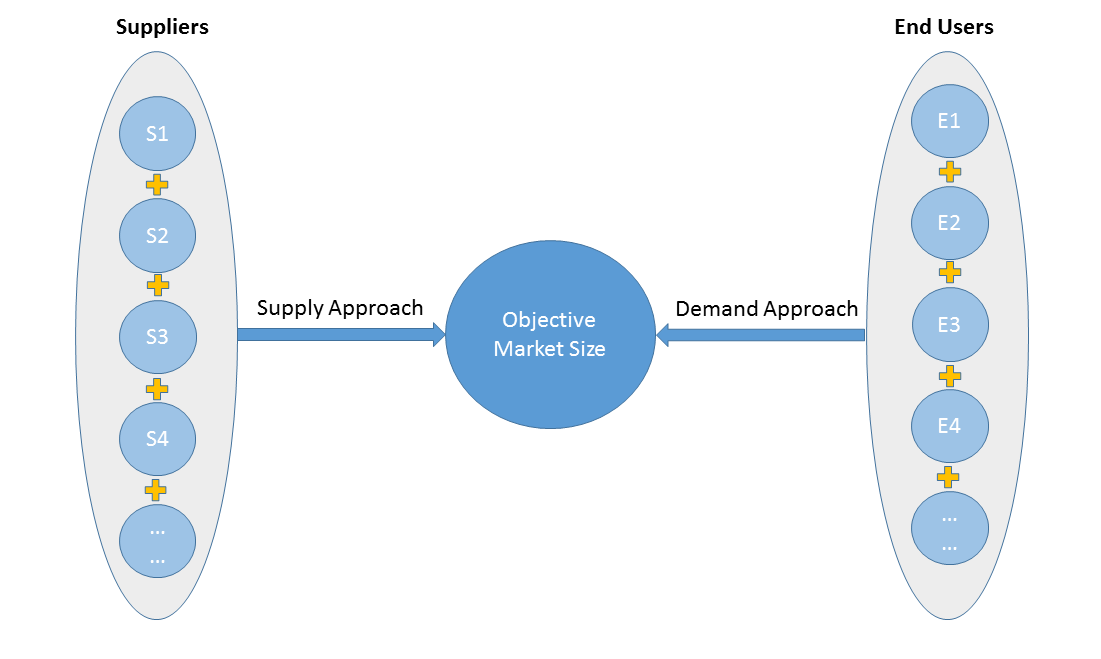
2)Supply & Demand Approach
Supply approach is based on assessments of the size of each competitor supplying the objective market.
Demand approach combine end-user data within a market to estimate the objective market size. It is sometimes referred to as bottom-up approach.
- Forecasting Methodology
- Numerous factors impacting the market trend are considered for forecast model:
- New technology and application in the future;
- New project planned/under contraction;
- Global and regional underlying economic growth;
- Threatens of substitute products;
- Industry expert opinion;
- Policy and Society implication.
- Analysis Tools
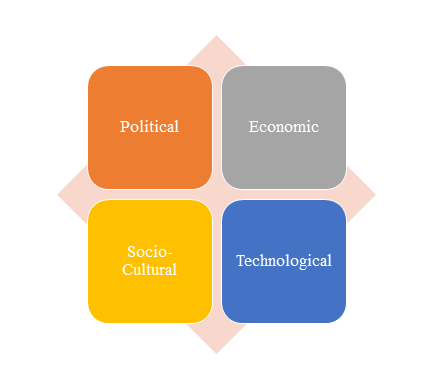
1)PEST Analysis
PEST Analysis is a simple and widely used tool that helps our client analyze the Political, Economic, Socio-Cultural, and Technological changes in their business environment.
- Benefits of a PEST analysis:
- It helps you to spot business opportunities, and it gives you advanced warning of significant threats.
- It reveals the direction of change within your business environment. This helps you shape what you’re doing, so that you work with change, rather than against it.
- It helps you avoid starting projects that are likely to fail, for reasons beyond your control.
- It can help you break free of unconscious assumptions when you enter a new country, region, or market; because it helps you develop an objective view of this new environment.
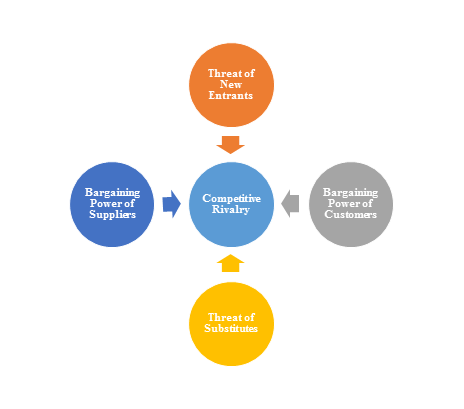
2)Porter’s Five Force Model Analysis
The Porter’s Five Force Model is a tool that can be used to analyze the opportunities and overall competitive advantage. The five forces that can assist in determining the competitive intensity and potential attractiveness within a specific area.
- Threat of New Entrants: Profitable industries that yield high returns will attract new firms.
- Threat of Substitutes: A substitute product uses a different technology to try to solve the same economic need.
- Bargaining Power of Customers: the ability of customers to put the firm under pressure, which also affects the customer's sensitivity to price changes.
- Bargaining Power of Suppliers: Suppliers of raw materials, components, labor, and services (such as expertise) to the firm can be a source of power over the firm when there are few substitutes.
- Competitive Rivalry: For most industries the intensity of competitive rivalry is the major determinant of the competitiveness of the industry.
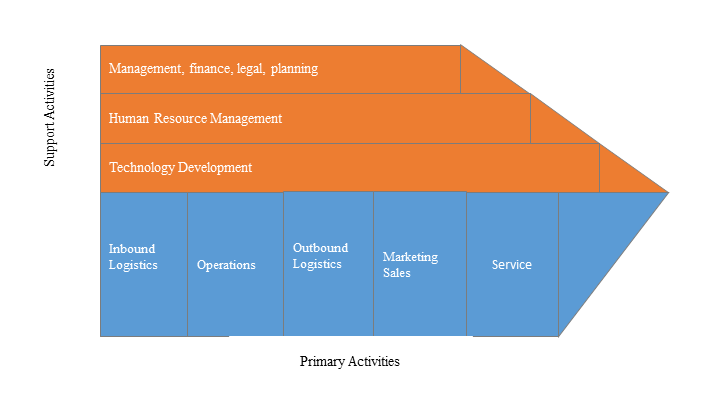
3)Value Chain Analysis
Value chain analysis is a tool to identify activities, within and around the firm and relating these activities to an assessment of competitive strength. Value chain can be analyzed by primary activities and supportive activities. Primary activities include: inbound logistics, operations, outbound logistics, marketing & sales, service. Support activities include: technology development, human resource management, management, finance, legal, planning.
4)SWOT Analysis
SWOT analysis is a tool used to evaluate a company's competitive position by identifying its strengths, weaknesses, opportunities and threats. The strengths and weakness is the inner factor; the opportunities and threats are the external factor. By analyzing the inner and external factors, the analysis can provide the detail information of the position of a player and the characteristics of the industry.
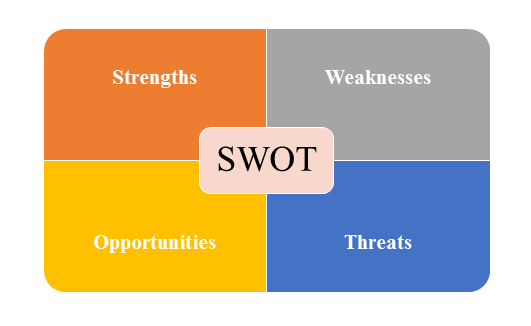
- Strengths describe what the player excels at and separates it from the competition
- Weaknesses stop the player from performing at its optimum level.
- Opportunities refer to favorable external factors that the player can use to give it a competitive advantage.
- Threats refer to factors that have the potential to harm the player.
- Data Sources
| Primary Sources | Secondary Sources |
|---|---|
| Face to face/Phone Interviews with market participants, such as: Manufactures; Distributors; End-users; Experts. Online Survey |
Government/International Organization Data: Annual Report/Presentation/Fact Book Internet Source Information Industry Association Data Free/Purchased Database Market Research Report Book/Journal/News |