Blood Collection Devices Market Insights 2025, Analysis and Forecast to 2030, by Manufacturers, Regions, Technology, Application, Product Type
- Single User License (1 Users) $ 3,500
- Team License (2~5 Users) $ 4,500
- Corporate License (>5 Users) $ 5,500
Blood collection devices encompass a comprehensive range of medical instruments and consumables designed to obtain blood specimens safely, efficiently, and reliably for diagnostic testing, therapeutic procedures, blood banking, and research applications. These essential healthcare tools include evacuated blood collection tubes with various anticoagulants and additives optimized for specific laboratory analyses, hypodermic needles and safety-engineered needles minimizing needlestick injuries and infectious disease transmission, syringes enabling manual aspiration and injection, butterfly needles facilitating venipuncture in challenging patient populations, blood collection sets for larger volume donations and therapeutic phlebotomy, lancets for capillary blood sampling, and specialized devices including arterial blood gas samplers and pediatric collection systems. The technology serves the fundamental healthcare function of obtaining biological specimens that inform the vast majority of clinical decisions, with laboratory test results influencing an estimated seventy percent of medical diagnoses and treatment monitoring decisions. Modern blood collection devices incorporate sophisticated engineering addressing safety, specimen quality, patient comfort, healthcare worker protection, and workflow efficiency. Safety-engineered devices with retractable needles or protective shields respond to occupational safety regulations reducing needlestick injuries that pose hepatitis and HIV transmission risks. Closed collection systems minimize specimen contamination and hemolysis that compromise test accuracy. Color-coded tube stoppers standardize laboratory workflows and reduce collection errors. The market reflects healthcare's fundamental dependence on diagnostic testing, evolving toward safer technologies, point-of-care applications, and systems supporting precision medicine requiring increasingly specialized specimen collection protocols.
The global blood collection devices market is estimated to reach approximately USD 4.0 billion to USD 10.0 billion by 2025, with the substantial range reflecting different definitional boundaries regarding included product categories, geographic coverage, and whether valuations encompass only blood collection tubes and basic needles or extend to comprehensive blood drawing and donation systems, automated collection devices, and accessories. This significant market size underscores the universal and indispensable nature of blood collection in healthcare delivery across every therapeutic area and healthcare setting. Between 2025 and 2030, the market is projected to grow at a compound annual growth rate ranging from 5.0% to 15.0%, indicating robust expansion driven by aging populations requiring more frequent diagnostic testing, chronic disease prevalence increasing monitoring needs, preventive healthcare emphasis promoting routine screenings, emerging market healthcare infrastructure development, safety device adoption driven by regulations and occupational health priorities, and technological innovations including specialized collection systems supporting advanced diagnostics. The wide growth corridor reflects divergent scenarios regarding healthcare spending trajectories across different economic regions, the pace of safety device adoption particularly in cost-sensitive markets, competitive pricing dynamics as patents expire and biosimilar manufacturers enter markets, and success of premium specialized collection systems commanding higher prices versus commodity pressures in standard products. Higher growth projections assume aggressive healthcare infrastructure expansion in developing regions, mandatory safety device regulations proliferating globally, successful commercialization of specialized collection systems for liquid biopsy and molecular diagnostics, and sustained diagnostic testing volume growth. Conservative estimates account for healthcare cost containment pressures, generic competition compressing prices, market saturation in developed regions, and potential disruptive technologies including non-invasive diagnostic approaches reducing blood collection needs.
Industry Characteristics
The blood collection devices industry operates within the broader medical device sector, characterized by stringent regulatory requirements, hospital and laboratory purchasing patterns, emphasis on safety and quality, and diverse product portfolios addressing varied clinical needs and economic segments. The market exhibits mature technology in conventional products including standard blood collection tubes and basic needles, where incremental improvements in safety, ergonomics, and manufacturing efficiency characterize innovation, while simultaneously demonstrating substantial innovation in specialized applications including liquid biopsy collection for circulating tumor cell analysis, cell-free DNA preservation, metabolomics specimen handling, and point-of-care collection systems.
Product standardization represents a defining industry characteristic enabling interoperability across healthcare systems. The International Organization for Standardization and Clinical and Laboratory Standards Institute establish specifications for blood collection tubes, needles, and procedures ensuring consistent performance and compatibility. Color-coded tube stoppers following standardized schemes enable healthcare workers to select appropriate tubes for ordered tests, reducing errors and streamlining workflows. Standardized needle gauges, hub designs, and connection fittings ensure compatibility with various collection devices and transfer systems. This standardization creates efficiencies but also intensifies price competition as products become commoditized when performance differences narrow.
Safety engineering transforms the industry responding to needlestick injury concerns affecting healthcare workers. Safety-engineered needles incorporating shielding mechanisms, retractable needles withdrawing into holders after use, and blunting technology rendering needles unusable after collection substantially reduce injury risks. Regulatory mandates including the U.S. Needlestick Safety and Prevention Act and European Union Directive on sharps injuries drive adoption despite higher costs compared to conventional needles. This regulatory environment creates competitive advantages for manufacturers with comprehensive safety portfolios and innovation capabilities while challenging smaller producers lacking resources to develop compliant products.
The market demonstrates consumable product characteristics with single-use devices generating recurring revenue proportional to diagnostic testing volumes rather than durable equipment replacement cycles. This creates stable, predictable demand correlated with patient encounters, diagnostic test ordering patterns, and blood donation activities. Healthcare providers maintain ongoing purchasing relationships with suppliers through group purchasing organization contracts, hospital system agreements, and laboratory distribution channels. Pricing negotiations balance product quality, reliability, safety features, service levels, and total cost considerations.
Quality and reliability assume paramount importance as specimen collection directly impacts diagnostic accuracy. Hemolysis, contamination, improper anticoagulation, and delayed processing compromise test results potentially leading to incorrect diagnoses, unnecessary testing, and adverse patient outcomes. Manufacturers invest substantially in quality control, validation studies, and supply chain management ensuring consistent product performance. Regulatory scrutiny including U.S. Food and Drug Administration oversight, European Conformité Européenne marking requirements, and various national regulatory approvals creates barriers to entry protecting established manufacturers while ensuring product safety and efficacy.
The industry serves diverse customer segments with different priorities and purchasing behaviors. Large hospital systems and integrated delivery networks negotiate volume contracts emphasizing total cost, standardization across facilities, and supplier reliability. Independent hospitals and small hospital groups may purchase through group purchasing organizations or regional distributors. Commercial reference laboratories including Quest Diagnostics and Laboratory Corporation of America process millions of specimens annually, wielding substantial purchasing leverage and demanding reliable, cost-effective products. Physician office laboratories, outpatient clinics, and urgent care centers represent fragmented customers typically purchasing through distributors. Blood collection organizations including American Red Cross and regional blood banks require specialized collection sets and anticoagulant formulations for donation processing. Each segment demonstrates different decision criteria balancing clinical performance, safety, cost, and convenience.
Regional Market Trends
Blood collection device demand and market development demonstrate geographic variations reflecting healthcare infrastructure maturity, regulatory frameworks, disease prevalence, diagnostic testing rates, and economic development levels.
North America represents a substantial market share with projected growth ranging from 4.0% to 10.0% through 2030. The United States drives regional demand through sophisticated healthcare infrastructure, high diagnostic testing utilization, aging population requiring chronic disease monitoring, comprehensive insurance coverage supporting diagnostic services, and stringent occupational safety regulations mandating safety-engineered devices. The Needlestick Safety and Prevention Act requirements accelerated safety device adoption, creating market transition toward premium-priced products. Large commercial laboratories, hospital systems, and physician practices generate substantial recurring demand. However, market maturity, healthcare cost containment pressures affecting diagnostic reimbursement, and consolidation among healthcare purchasers creating negotiating leverage moderate growth and pressure pricing. Generic competition in standard products and patent expirations intensify price competition. Canada contributes to regional demand through publicly funded healthcare supporting diagnostic services, though smaller population and cost-conscious procurement practices influence market characteristics.
Europe constitutes another major market with estimated growth in the range of 3.5% to 9.0% over the forecast period. The region's diverse healthcare systems exhibit varied characteristics with Western European countries demonstrating mature markets while Eastern European nations show higher growth from infrastructure development and rising healthcare access. Germany, France, United Kingdom, Italy, and Spain represent largest national markets supported by aging populations, universal healthcare coverage, and comprehensive diagnostic capabilities. The European Union's Sharps Directive mandating safety-engineered devices drives market transition similar to North American experiences, though implementation timelines and enforcement rigor vary across member states. The region's emphasis on healthcare cost-effectiveness, competitive tendering processes, and price controls in some countries create pricing pressures. However, quality expectations, regulatory compliance requirements, and patient safety priorities support premium product adoption where performance advantages justify costs. Brexit impacts complicate regulatory pathways and supply chains affecting market dynamics in the United Kingdom.
Asia-Pacific emerges as the fastest-growing regional market, with projected growth rates ranging from 7.0% to 18.0% CAGR through 2030, driven by enormous populations, rapid economic development, healthcare infrastructure expansion, rising middle classes demanding quality healthcare, increasing health insurance penetration, and growing chronic disease burden from aging demographics and lifestyle changes. China represents the largest opportunity through massive population, expanding hospital networks, growing diagnostic testing volumes, increasing healthcare expenditure, and government healthcare reforms improving access. Domestic manufacturers including Improve Medical and Tuo Kang Medical compete with international suppliers, offering cost-competitive products particularly in standard categories while international brands maintain advantages in premium and specialized segments. Japan demonstrates mature market characteristics with aging population driving diagnostic demand, sophisticated healthcare system, quality preferences, and comprehensive insurance coverage, though economic stagnation and demographic decline moderate growth. India shows substantial potential through large population, expanding private healthcare sector, medical tourism, increasing diagnostic awareness, and government health insurance schemes, though price sensitivity, fragmented healthcare delivery, and infrastructure constraints affect premium product penetration. Southeast Asian nations including Indonesia, Thailand, Vietnam, and Philippines demonstrate emerging markets with healthcare infrastructure development and rising incomes supporting demand growth. Australia and New Zealand represent developed markets with mature healthcare systems and stable demand patterns.
Latin America remains a smaller market with projected growth in the range of 4.5% to 12.0%. Brazil and Mexico drive regional demand through large populations, expanding healthcare access, growing middle classes, and improving diagnostic infrastructure. Public healthcare systems represent substantial purchasers though budget constraints and procurement complexities affect spending patterns. Private healthcare sectors in major cities adopt international standards and quality products. However, economic volatility, currency fluctuations, import dependencies, and healthcare financing challenges create uncertainties. Argentina, Chile, and Colombia contribute to regional demand. Price sensitivity drives significant generic and locally manufactured product utilization. Nevertheless, urbanization, chronic disease prevalence, and gradual healthcare system strengthening support long-term market development.
The Middle East and Africa region demonstrates emerging potential with estimated growth ranging from 5.0% to 13.0%. Gulf Cooperation Council countries lead regional development through substantial healthcare investment, medical tourism initiatives, expatriate populations, and procurement of international-standard products for modern healthcare facilities. United Arab Emirates and Saudi Arabia invest heavily in healthcare infrastructure supporting diversification strategies. Israel demonstrates advanced healthcare capabilities and sophisticated diagnostic utilization. South Africa leads sub-Saharan Africa with established healthcare infrastructure, though public sector budget constraints and private sector fragmentation affect market characteristics. Much of Africa faces healthcare infrastructure deficits, limited diagnostic capabilities, affordability barriers, and supply chain challenges restricting market development. International development programs and disease-specific initiatives including HIV and malaria programs create specialized demand. However, quality assurance concerns, counterfeit products, and variable regulatory enforcement complicate markets in some regions.
Application Analysis
Hospitals and clinics constitute the largest application segment with projected growth of 5.0% to 14.0% CAGR through 2030. This diverse category encompasses inpatient acute care hospitals performing emergency diagnostics and treatment monitoring, outpatient clinics providing primary care and specialty services, urgent care centers offering convenient acute care access, ambulatory surgery centers requiring pre-operative testing, and dialysis centers conducting routine monitoring. Hospital laboratories process thousands of daily specimens across chemistry, hematology, immunology, microbiology, and molecular diagnostics, generating substantial blood collection device consumption. Emergency departments require rapid turnaround testing for trauma, cardiac events, and acute illnesses. Critical care units monitor patients continuously requiring frequent blood sampling. Oncology departments administer chemotherapy requiring monitoring for toxicity and efficacy. The aging population requiring hospitalization and chronic disease management drives increasing inpatient diagnostic volumes. Outpatient care shifts reduce hospital days but maintain or increase diagnostic testing as monitoring moves to ambulatory settings. This segment demonstrates diverse product needs spanning pediatric to geriatric populations, requiring various collection volumes and specialized handling. Safety device adoption reaches highest levels in hospital settings given occupational health priorities and regulatory compliance. However, hospital cost pressures, value-based care models emphasizing efficiency, and competitive contracting create pricing sensitivities.
Diagnostic and pathology centers represent a substantial and growing segment with estimated growth of 5.5% to 16.0% over the forecast period. Commercial reference laboratories including national chains and regional providers process millions of specimens from physicians' offices, clinics, hospitals, and direct-to-consumer services. These high-volume, centralized facilities emphasize efficiency, automation compatibility, specimen quality, and cost-effectiveness. Standardization across massive testing volumes creates operational efficiencies and purchasing leverage. Pathology laboratories performing anatomic and clinical pathology serve hospital systems and independent physicians. Specialized laboratories focusing on genetics, molecular diagnostics, toxicology, and esoteric testing demonstrate rapid growth driven by precision medicine, pharmacogenomics, and advanced diagnostics. Point-of-care testing expansion in retail clinics, pharmacy-based health services, and workplace health settings creates demand for simplified collection systems and integrated testing devices. Direct-to-consumer testing services offering wellness panels and disease screening represent emerging channels. This segment benefits from diagnostic testing volume growth outpacing healthcare utilization generally, driven by preventive care emphasis, guideline-recommended screenings, chronic disease monitoring, and advanced diagnostic capabilities expanding test menus. However, reimbursement pressures, consolidation creating purchasing concentration, and laboratory developed test competition affect market dynamics.
Blood banks and transfusion services demonstrate specialized requirements with projected growth of 4.5% to 12.0% through 2030. This segment includes blood collection organizations managing donor recruitment and whole blood collection, hospital-based transfusion services storing and dispensing blood products, plasma collection centers supplying pharmaceutical manufacturers with raw materials for therapeutic protein production, and cord blood banks preserving stem cells. Blood donation requires specialized collection sets with anticoagulant solutions optimized for component separation and storage, larger collection volumes compared to diagnostic sampling, and closed systems minimizing contamination risks. Plasma collection for fractionation utilizes automated apheresis equipment and specialized disposables. Regulatory requirements for blood safety, donor screening, and product quality create stringent specifications. Aging populations and chronic diseases increase transfusion needs while declining donor rates from younger generations create ongoing tension between supply and demand. Plasma-derived therapeutics for immunodeficiencies, hemophilia, and other conditions drive plasma collection demand. However, blood conservation strategies, surgical technique improvements, and pharmaceutical alternatives reduce transfusion needs in some clinical scenarios. This specialized segment demonstrates lower growth compared to diagnostic applications but maintains importance given essential services.
Other applications include research laboratories, veterinary medicine, forensic and toxicology services, drug development clinical trials, occupational health screening, wellness and preventive medicine programs, and home healthcare sampling. This diverse category demonstrates collective growth of 4.0% to 11.0%. Research applications support biomedical investigation, pharmaceutical research, and clinical studies requiring biospecimen collection. Veterinary medicine increasingly adopts human healthcare diagnostic capabilities for companion animals. Home healthcare services including visiting nurses and mobile phlebotomy provide convenient collection for elderly, disabled, or geographically isolated patients. Workplace wellness programs offer health screening and chronic disease management. The breadth and diversity of applications beyond traditional clinical settings create incremental demand supporting overall market expansion.
Type Analysis
Blood collection tubes represent the largest product category with estimated growth of 5.0% to 14.0% CAGR through 2030. This diverse segment encompasses evacuated tubes with vacuum-sealed stoppers drawing predetermined blood volumes through needle puncture, various tube types optimized for different tests including serum tubes for chemistry and immunoassays, plasma tubes with anticoagulants for coagulation and molecular testing, whole blood tubes for hematology, and specialized tubes for trace elements, glucose preservation, and molecular diagnostics. Tube additives including clot activators, anticoagulants such as EDTA, heparin, and citrate, and specialized preservatives optimize specimen handling for specific analyses. Color-coded stoppers following standardized schemes enable proper test-specific tube selection. Tube sizes range from micro-collection volumes for pediatric and capillary sampling to standard adult collection volumes. Manufacturing involves glass or plastic tubes, rubber or synthetic stoppers, vacuum processing, and additive preparation requiring quality control ensuring consistent performance. Innovation focuses on specialized applications including cell-free DNA preservation tubes for liquid biopsy enabling non-invasive cancer monitoring, metabolomics stabilization tubes maintaining metabolite profiles, and RNA preservation tubes supporting gene expression studies. Safety features including safety caps and needleless transfer devices reduce occupational exposure. This segment benefits from diagnostic testing volume growth and expanding test menus requiring specialized collection. However, commoditization of standard tubes creates intense price competition while specialized tubes command premium pricing.
Needles and syringes constitute another major category with projected growth of 5.0% to 15.0% over the forecast period. This segment includes hypodermic needles in various gauges from fine 23-25 gauge for routine adult venipuncture to larger bore for blood donation, safety-engineered needles with protective shields or retraction mechanisms, butterfly or winged infusion sets providing flexible tubing and stability for difficult draws, syringes for manual aspiration ranging from 1ml to 60ml capacities, and specialized arterial puncture needles for blood gas sampling. Needle manufacturing requires precision fabrication achieving sharpness minimizing insertion pain while maintaining wall thickness providing structural integrity and flow rates. Bevel design, lubricant coatings, and wall engineering influence puncture force and patient comfort. Safety engineering represents the primary innovation focus with passive safety mechanisms activating automatically after use, active mechanisms requiring healthcare worker activation, and various shield, retraction, and blunting technologies. Regulatory requirements, occupational safety priorities, and litigation risks surrounding needlestick injuries drive safety device adoption despite cost premiums ranging from thirty to one hundred percent compared to conventional needles. This segment benefits from universal need across medical procedures, safety device mandates expanding globally, and insulin injection requirements from diabetes prevalence. However, manufacturing commoditization, patent expirations, and intense competition pressure pricing particularly for standard products.
Other devices include lancets for capillary blood sampling used in glucose monitoring and point-of-care testing, blood collection sets combining needles with tubing and collection vessels for donations and therapeutic phlebotomy, pediatric and neonatal specialized collection systems minimizing collection volumes and trauma, arterial blood gas sampling kits pre-heparinized and sealed for immediate analysis, transfer devices enabling closed transfer from syringes to collection tubes without needle exposure, blood culture collection systems maintaining sterility and supporting automated processing, and accessories including tourniquet, bandages, disinfectants, and holders. This diverse category demonstrates projected growth of 4.5% to 12.0% through 2030. Lancets benefit from diabetes prevalence and home glucose monitoring. Specialized pediatric systems serve vulnerable populations where minimizing blood volumes and procedure trauma proves critical. Arterial sampling addresses critical care and surgical monitoring needs. Transfer devices enhance safety and specimen quality. Accessories represent lower-margin commodity products but create comprehensive portfolio completeness. Innovation opportunities exist in devices supporting difficult venous access, patient comfort improvements, and integration with automated laboratory systems.
Company Landscape
The blood collection devices market engages established medical device manufacturers with extensive healthcare portfolios alongside specialized companies focused on specimen collection and laboratory products.
Becton Dickinson (BD), an American medical technology company, dominates the global market with comprehensive product portfolio spanning BD Vacutainer blood collection tubes representing the most recognized brand worldwide, BD Eclipse and BD SafetyGlide safety needles, syringes, and complete blood collection systems. The company's market leadership derives from extensive distribution networks, hospital relationships, manufacturing scale, continuous innovation, and quality reputation. BD's acquisition of C.R. Bard and Carefusion expanded its medical device portfolio though blood collection remains a core business. Global manufacturing footprint and regulatory expertise support market access across regions.
Terumo Corporation, a Japanese medical device manufacturer, offers comprehensive blood collection and infusion products including Venoject collection tubes, Terumo needles and syringes, and specialized devices. The company's precision manufacturing, quality reputation, and strong Asian market presence create competitive positioning. Diversified product portfolio spanning cardiovascular, general hospital, and blood management products provides scale and customer relationships.
Greiner Bio-One, an Austrian company specializing in laboratory and medical products, provides Vacuette blood collection tubes and related systems serving diagnostic laboratories and healthcare facilities. Focus on quality, innovation, and customer service differentiates the company in competitive markets. Strong European presence and expanding international footprint support growth.
Sarstedt AG, a German family-owned medical device company, manufactures S-Monovette blood collection systems offering unique syringe-vacuum dual functionality, standard tubes, and comprehensive phlebotomy products. The company's innovation, quality standards, and European market strength create solid positioning. Direct sales model and customer relationships enable service emphasis.
Medtronic, while primarily known for cardiovascular devices and surgical equipment, participates in blood management through specialized coagulation monitoring and blood conservation technologies complementing broader procedural offerings.
Haemonetics specializes in blood management and transfusion medicine, providing automated blood collection systems for plasma and platelet apheresis, blood processing equipment, and related disposables serving blood banks and plasma collection centers. Focus on automated collection and blood management rather than manual diagnostic sampling differentiates positioning.
Fresenius Kabi, a German healthcare company, operates within blood collection primarily through apheresis products and transfusion technologies serving blood banks and plasma centers supporting its pharmaceutical plasma derivatives business.
Nipro Corporation, a Japanese medical device and pharmaceutical company, manufactures needles, syringes, blood collection tubes, and dialysis products serving Asian and global markets. Diversified product portfolio and cost-competitive manufacturing support market presence.
Kawasumi Laboratories, another Japanese medical device specialist, provides blood collection and transfusion products leveraging manufacturing expertise and Asian market relationships.
Narang Medical, an Indian manufacturer, produces blood collection tubes, needles, and laboratory consumables serving domestic and export markets. Cost-competitive positioning addresses price-sensitive segments while quality improvements target international market expansion.
Improve Medical and Tuo Kang Medical, Chinese manufacturers, demonstrate emerging market capabilities producing blood collection tubes and needles serving domestic demand and expanding international presence through competitive pricing and improving quality.
Hindustan Syringes and Medical Devices, one of India's largest syringe manufacturers, produces safety syringes and needles serving domestic healthcare needs and export markets, emphasizing affordability and manufacturing scale.
Sol-Millennium and Vitrex Medical represent additional regional players contributing to market competition through specialized products, geographic focus, or cost positioning addressing segments underserved by multinational leaders.
Value Chain Analysis
The blood collection devices value chain encompasses multiple stages from raw materials through clinical utilization and specimen processing.
Raw material sourcing provides glass or medical-grade plastics for tubes, stainless steel for needles, natural or synthetic rubber for stoppers, chemical additives including anticoagulants and clot activators, and packaging materials. Material quality directly impacts product performance, biocompatibility, and shelf life. Supply chain reliability and cost management represent critical capabilities.
Component manufacturing produces glass tubes through molding and annealing, plastic tubes via injection molding or extrusion, needles through precision grinding and sharpening, stoppers via molding and compounding, and additive formulations requiring pharmaceutical-grade processing. Manufacturing precision, quality control, and process validation ensure consistent product performance meeting regulatory specifications.
Device assembly and finishing integrates components into finished products including evacuated tube preparation, needle assembly with hubs and safety mechanisms, syringe assembly, and final product preparation. Automated assembly lines achieve high-volume production with consistent quality. Sterilization via ethylene oxide, gamma radiation, or other methods ensures sterility. Quality testing validates specifications before release.
Packaging and labeling protects products during distribution and communicates usage information including expiration dating, lot traceability, instructions, and regulatory compliance information. Packaging design balances protection, convenience, and cost while supporting supply chain efficiency.
Distribution channels move products from manufacturing to end users through direct sales to large hospital systems and laboratory chains, medical-surgical distributors serving hospitals and clinics, group purchasing organizations negotiating contracts on behalf of member healthcare facilities, international distributors managing import, regulatory compliance, and local distribution in various countries, and specialize
Chapter 1 Executive Summary
Chapter 2 Abbreviation and Acronyms
Chapter 3 Preface
3.1 Research Scope
3.2 Research Sources
3.2.1 Data Sources
3.2.2 Assumptions
3.3 Research Method
Chapter 4 Market Landscape
4.1 Market Overview
4.2 Classification/Types
4.3 Application/End Users
Chapter 5 Market Trend Analysis
5.1 introduction
5.2 Drivers
5.3 Restraints
5.4 Opportunities
5.5 Threats
Chapter 6 industry Chain Analysis
6.1 Upstream/Suppliers Analysis
6.2 Blood Collection Devices Analysis
6.2.1 Technology Analysis
6.2.2 Cost Analysis
6.2.3 Market Channel Analysis
6.3 Downstream Buyers/End Users
Chapter 7 Latest Market Dynamics
7.1 Latest News
7.2 Merger and Acquisition
7.3 Planned/Future Project
7.4 Policy Dynamics
Chapter 8 Historical and Forecast Blood Collection Devices Market in North America (2020-2030)
8.1 Blood Collection Devices Market Size
8.2 Blood Collection Devices Market by End Use
8.3 Competition by Players/Suppliers
8.4 Blood Collection Devices Market Size by Type
8.5 Key Countries Analysis
8.5.1 United States
8.5.2 Canada
8.5.3 Mexico
Chapter 9 Historical and Forecast Blood Collection Devices Market in South America (2020-2030)
9.1 Blood Collection Devices Market Size
9.2 Blood Collection Devices Market by End Use
9.3 Competition by Players/Suppliers
9.4 Blood Collection Devices Market Size by Type
9.5 Key Countries Analysis
9.5.1 Brazil
9.5.2 Argentina
9.5.3 Chile
9.5.4 Peru
Chapter 10 Historical and Forecast Blood Collection Devices Market in Asia & Pacific (2020-2030)
10.1 Blood Collection Devices Market Size
10.2 Blood Collection Devices Market by End Use
10.3 Competition by Players/Suppliers
10.4 Blood Collection Devices Market Size by Type
10.5 Key Countries Analysis
10.5.1 China
10.5.2 India
10.5.3 Japan
10.5.4 South Korea
10.5.5 Southest Asia
10.5.6 Australia
Chapter 11 Historical and Forecast Blood Collection Devices Market in Europe (2020-2030)
11.1 Blood Collection Devices Market Size
11.2 Blood Collection Devices Market by End Use
11.3 Competition by Players/Suppliers
11.4 Blood Collection Devices Market Size by Type
11.5 Key Countries Analysis
11.5.1 Germany
11.5.2 France
11.5.3 United Kingdom
11.5.4 Italy
11.5.5 Spain
11.5.6 Belgium
11.5.7 Netherlands
11.5.8 Austria
11.5.9 Poland
11.5.10 Russia
Chapter 12 Historical and Forecast Blood Collection Devices Market in MEA (2020-2030)
12.1 Blood Collection Devices Market Size
12.2 Blood Collection Devices Market by End Use
12.3 Competition by Players/Suppliers
12.4 Blood Collection Devices Market Size by Type
12.5 Key Countries Analysis
12.5.1 Egypt
12.5.2 Israel
12.5.3 South Africa
12.5.4 Gulf Cooperation Council Countries
12.5.5 Turkey
Chapter 13 Summary For Global Blood Collection Devices Market (2020-2025)
13.1 Blood Collection Devices Market Size
13.2 Blood Collection Devices Market by End Use
13.3 Competition by Players/Suppliers
13.4 Blood Collection Devices Market Size by Type
Chapter 14 Global Blood Collection Devices Market Forecast (2025-2030)
14.1 Blood Collection Devices Market Size Forecast
14.2 Blood Collection Devices Application Forecast
14.3 Competition by Players/Suppliers
14.4 Blood Collection Devices Type Forecast
Chapter 15 Analysis of Global Key Vendors
15.1 Becton Dickinson (BD)
15.1.1 Company Profile
15.1.2 Main Business and Blood Collection Devices Information
15.1.3 SWOT Analysis of Becton Dickinson (BD)
15.1.4 Becton Dickinson (BD) Blood Collection Devices Sales, Revenue, Price and Gross Margin (2020-2025)
15.2 Terumo Corporation
15.2.1 Company Profile
15.2.2 Main Business and Blood Collection Devices Information
15.2.3 SWOT Analysis of Terumo Corporation
15.2.4 Terumo Corporation Blood Collection Devices Sales, Revenue, Price and Gross Margin (2020-2025)
15.3 Greiner Bio-One
15.3.1 Company Profile
15.3.2 Main Business and Blood Collection Devices Information
15.3.3 SWOT Analysis of Greiner Bio-One
15.3.4 Greiner Bio-One Blood Collection Devices Sales, Revenue, Price and Gross Margin (2020-2025)
15.4 Sarstedt AG
15.4.1 Company Profile
15.4.2 Main Business and Blood Collection Devices Information
15.4.3 SWOT Analysis of Sarstedt AG
15.4.4 Sarstedt AG Blood Collection Devices Sales, Revenue, Price and Gross Margin (2020-2025)
15.5 Medtronic
15.5.1 Company Profile
15.5.2 Main Business and Blood Collection Devices Information
15.5.3 SWOT Analysis of Medtronic
15.5.4 Medtronic Blood Collection Devices Sales, Revenue, Price and Gross Margin (2020-2025)
15.6 Haemonetics
15.6.1 Company Profile
15.6.2 Main Business and Blood Collection Devices Information
15.6.3 SWOT Analysis of Haemonetics
15.6.4 Haemonetics Blood Collection Devices Sales, Revenue, Price and Gross Margin (2020-2025)
15.7 Fresenius Kabi
15.7.1 Company Profile
15.7.2 Main Business and Blood Collection Devices Information
15.7.3 SWOT Analysis of Fresenius Kabi
15.7.4 Fresenius Kabi Blood Collection Devices Sales, Revenue, Price and Gross Margin (2020-2025)
15.8 Nipro Corporation
15.8.1 Company Profile
15.8.2 Main Business and Blood Collection Devices Information
15.8.3 SWOT Analysis of Nipro Corporation
15.8.4 Nipro Corporation Blood Collection Devices Sales, Revenue, Price and Gross Margin (2020-2025)
15.9 Kawasumi Laboratories
15.9.1 Company Profile
15.9.2 Main Business and Blood Collection Devices Information
15.9.3 SWOT Analysis of Kawasumi Laboratories
15.9.4 Kawasumi Laboratories Blood Collection Devices Sales, Revenue, Price and Gross Margin (2020-2025)
15.10 Narang Medical
15.10.1 Company Profile
15.10.2 Main Business and Blood Collection Devices Information
15.10.3 SWOT Analysis of Narang Medical
15.10.4 Narang Medical Blood Collection Devices Sales, Revenue, Price and Gross Margin (2020-2025)
Please ask for sample pages for full companies list
Table Research Scope of Blood Collection Devices Report
Table Data Sources of Blood Collection Devices Report
Table Major Assumptions of Blood Collection Devices Report
Table Blood Collection Devices Classification
Table Blood Collection Devices Applications
Table Drivers of Blood Collection Devices Market
Table Restraints of Blood Collection Devices Market
Table Opportunities of Blood Collection Devices Market
Table Threats of Blood Collection Devices Market
Table Raw Materials Suppliers
Table Different Production Methods of Blood Collection Devices
Table Cost Structure Analysis of Blood Collection Devices
Table Key End Users
Table Latest News of Blood Collection Devices Market
Table Merger and Acquisition
Table Planned/Future Project of Blood Collection Devices Market
Table Policy of Blood Collection Devices Market
Table 2020-2030 North America Blood Collection Devices Market Size
Table 2020-2030 North America Blood Collection Devices Market Size by Application
Table 2020-2025 North America Blood Collection Devices Key Players Revenue
Table 2020-2025 North America Blood Collection Devices Key Players Market Share
Table 2020-2030 North America Blood Collection Devices Market Size by Type
Table 2020-2030 United States Blood Collection Devices Market Size
Table 2020-2030 Canada Blood Collection Devices Market Size
Table 2020-2030 Mexico Blood Collection Devices Market Size
Table 2020-2030 South America Blood Collection Devices Market Size
Table 2020-2030 South America Blood Collection Devices Market Size by Application
Table 2020-2025 South America Blood Collection Devices Key Players Revenue
Table 2020-2025 South America Blood Collection Devices Key Players Market Share
Table 2020-2030 South America Blood Collection Devices Market Size by Type
Table 2020-2030 Brazil Blood Collection Devices Market Size
Table 2020-2030 Argentina Blood Collection Devices Market Size
Table 2020-2030 Chile Blood Collection Devices Market Size
Table 2020-2030 Peru Blood Collection Devices Market Size
Table 2020-2030 Asia & Pacific Blood Collection Devices Market Size
Table 2020-2030 Asia & Pacific Blood Collection Devices Market Size by Application
Table 2020-2025 Asia & Pacific Blood Collection Devices Key Players Revenue
Table 2020-2025 Asia & Pacific Blood Collection Devices Key Players Market Share
Table 2020-2030 Asia & Pacific Blood Collection Devices Market Size by Type
Table 2020-2030 China Blood Collection Devices Market Size
Table 2020-2030 India Blood Collection Devices Market Size
Table 2020-2030 Japan Blood Collection Devices Market Size
Table 2020-2030 South Korea Blood Collection Devices Market Size
Table 2020-2030 Southeast Asia Blood Collection Devices Market Size
Table 2020-2030 Australia Blood Collection Devices Market Size
Table 2020-2030 Europe Blood Collection Devices Market Size
Table 2020-2030 Europe Blood Collection Devices Market Size by Application
Table 2020-2025 Europe Blood Collection Devices Key Players Revenue
Table 2020-2025 Europe Blood Collection Devices Key Players Market Share
Table 2020-2030 Europe Blood Collection Devices Market Size by Type
Table 2020-2030 Germany Blood Collection Devices Market Size
Table 2020-2030 France Blood Collection Devices Market Size
Table 2020-2030 United Kingdom Blood Collection Devices Market Size
Table 2020-2030 Italy Blood Collection Devices Market Size
Table 2020-2030 Spain Blood Collection Devices Market Size
Table 2020-2030 Belgium Blood Collection Devices Market Size
Table 2020-2030 Netherlands Blood Collection Devices Market Size
Table 2020-2030 Austria Blood Collection Devices Market Size
Table 2020-2030 Poland Blood Collection Devices Market Size
Table 2020-2030 Russia Blood Collection Devices Market Size
Table 2020-2030 MEA Blood Collection Devices Market Size
Table 2020-2030 MEA Blood Collection Devices Market Size by Application
Table 2020-2025 MEA Blood Collection Devices Key Players Revenue
Table 2020-2025 MEA Blood Collection Devices Key Players Market Share
Table 2020-2030 MEA Blood Collection Devices Market Size by Type
Table 2020-2030 Egypt Blood Collection Devices Market Size
Table 2020-2030 Israel Blood Collection Devices Market Size
Table 2020-2030 South Africa Blood Collection Devices Market Size
Table 2020-2030 Gulf Cooperation Council Countries Blood Collection Devices Market Size
Table 2020-2030 Turkey Blood Collection Devices Market Size
Table 2020-2025 Global Blood Collection Devices Market Size by Region
Table 2020-2025 Global Blood Collection Devices Market Size Share by Region
Table 2020-2025 Global Blood Collection Devices Market Size by Application
Table 2020-2025 Global Blood Collection Devices Market Share by Application
Table 2020-2025 Global Blood Collection Devices Key Vendors Revenue
Table 2020-2025 Global Blood Collection Devices Key Vendors Market Share
Table 2020-2025 Global Blood Collection Devices Market Size by Type
Table 2020-2025 Global Blood Collection Devices Market Share by Type
Table 2025-2030 Global Blood Collection Devices Market Size by Region
Table 2025-2030 Global Blood Collection Devices Market Size Share by Region
Table 2025-2030 Global Blood Collection Devices Market Size by Application
Table 2025-2030 Global Blood Collection Devices Market Share by Application
Table 2025-2030 Global Blood Collection Devices Key Vendors Revenue
Table 2025-2030 Global Blood Collection Devices Key Vendors Market Share
Table 2025-2030 Global Blood Collection Devices Market Size by Type
Table 2025-2030 Blood Collection Devices Global Market Share by Type
Figure Market Size Estimated Method
Figure Major Forecasting Factors
Figure Blood Collection Devices Picture
Figure 2020-2030 North America Blood Collection Devices Market Size and CAGR
Figure 2020-2030 South America Blood Collection Devices Market Size and CAGR
Figure 2020-2030 Asia & Pacific Blood Collection Devices Market Size and CAGR
Figure 2020-2030 Europe Blood Collection Devices Market Size and CAGR
Figure 2020-2030 MEA Blood Collection Devices Market Size and CAGR
Figure 2020-2025 Global Blood Collection Devices Market Size and Growth Rate
Figure 2025-2030 Global Blood Collection Devices Market Size and Growth Rate
Research Methodology
- Market Estimated Methodology:
Bottom-up & top-down approach, supply & demand approach are the most important method which is used by HDIN Research to estimate the market size.
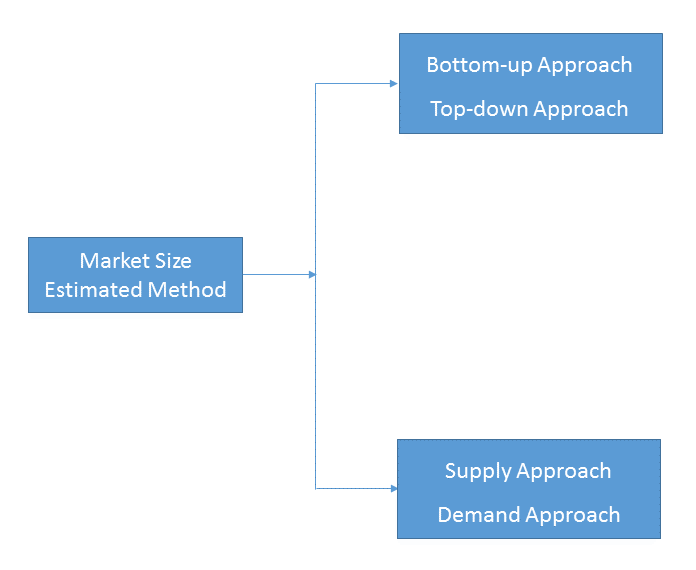
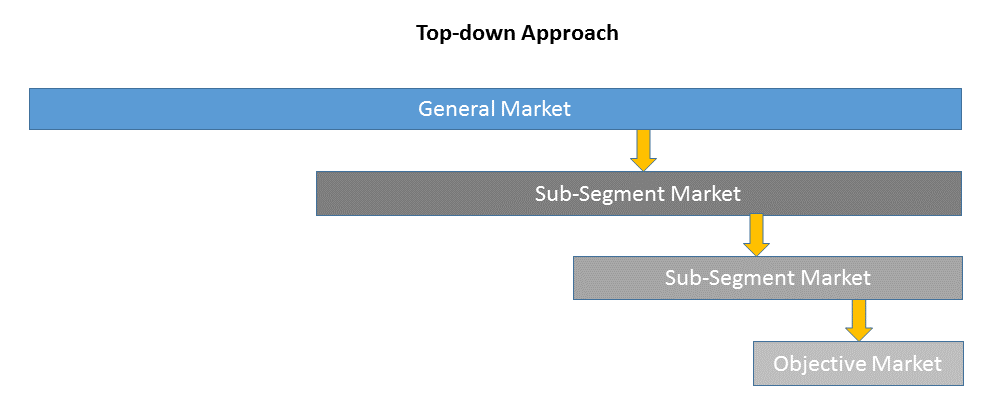
1)Top-down & Bottom-up Approach
Top-down approach uses a general market size figure and determines the percentage that the objective market represents.
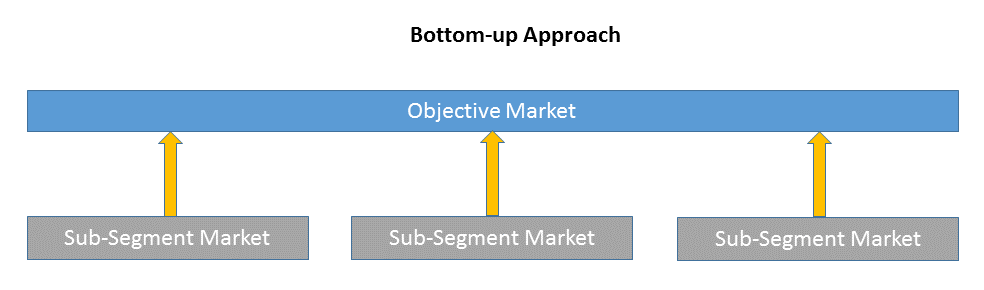
Bottom-up approach size the objective market by collecting the sub-segment information.
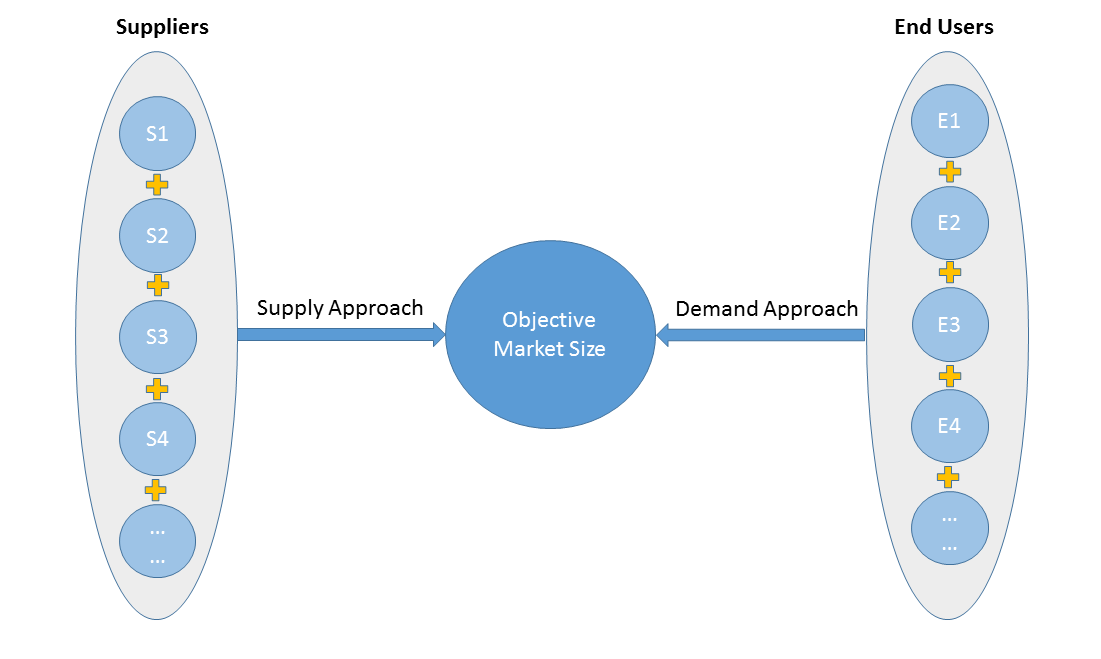
2)Supply & Demand Approach
Supply approach is based on assessments of the size of each competitor supplying the objective market.
Demand approach combine end-user data within a market to estimate the objective market size. It is sometimes referred to as bottom-up approach.
- Forecasting Methodology
- Numerous factors impacting the market trend are considered for forecast model:
- New technology and application in the future;
- New project planned/under contraction;
- Global and regional underlying economic growth;
- Threatens of substitute products;
- Industry expert opinion;
- Policy and Society implication.
- Analysis Tools
1)PEST Analysis
PEST Analysis is a simple and widely used tool that helps our client analyze the Political, Economic, Socio-Cultural, and Technological changes in their business environment.
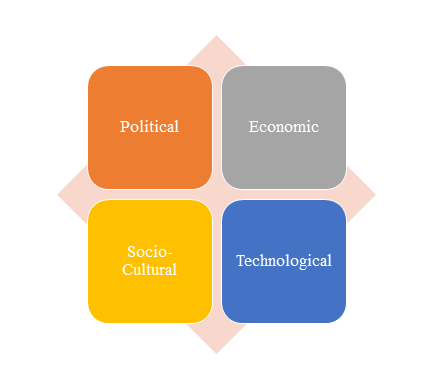
- Benefits of a PEST analysis:
- It helps you to spot business opportunities, and it gives you advanced warning of significant threats.
- It reveals the direction of change within your business environment. This helps you shape what you’re doing, so that you work with change, rather than against it.
- It helps you avoid starting projects that are likely to fail, for reasons beyond your control.
- It can help you break free of unconscious assumptions when you enter a new country, region, or market; because it helps you develop an objective view of this new environment.
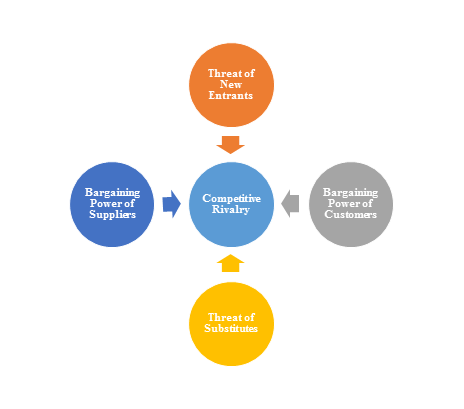
2)Porter’s Five Force Model Analysis
The Porter’s Five Force Model is a tool that can be used to analyze the opportunities and overall competitive advantage. The five forces that can assist in determining the competitive intensity and potential attractiveness within a specific area.
- Threat of New Entrants: Profitable industries that yield high returns will attract new firms.
- Threat of Substitutes: A substitute product uses a different technology to try to solve the same economic need.
- Bargaining Power of Customers: the ability of customers to put the firm under pressure, which also affects the customer's sensitivity to price changes.
- Bargaining Power of Suppliers: Suppliers of raw materials, components, labor, and services (such as expertise) to the firm can be a source of power over the firm when there are few substitutes.
- Competitive Rivalry: For most industries the intensity of competitive rivalry is the major determinant of the competitiveness of the industry.
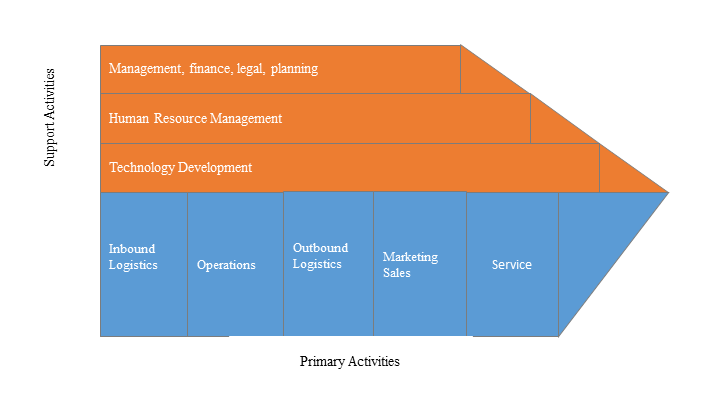
3)Value Chain Analysis
Value chain analysis is a tool to identify activities, within and around the firm and relating these activities to an assessment of competitive strength. Value chain can be analyzed by primary activities and supportive activities. Primary activities include: inbound logistics, operations, outbound logistics, marketing & sales, service. Support activities include: technology development, human resource management, management, finance, legal, planning.
4)SWOT Analysis
SWOT analysis is a tool used to evaluate a company's competitive position by identifying its strengths, weaknesses, opportunities and threats. The strengths and weakness is the inner factor; the opportunities and threats are the external factor. By analyzing the inner and external factors, the analysis can provide the detail information of the position of a player and the characteristics of the industry.
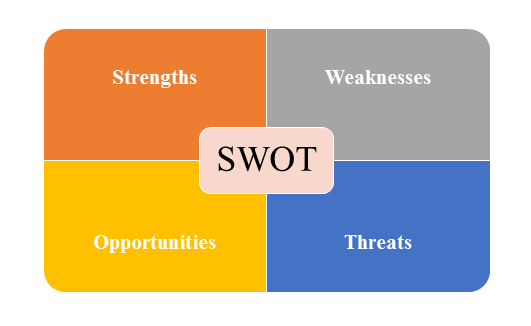
- Strengths describe what the player excels at and separates it from the competition
- Weaknesses stop the player from performing at its optimum level.
- Opportunities refer to favorable external factors that the player can use to give it a competitive advantage.
- Threats refer to factors that have the potential to harm the player.
- Data Sources
| Primary Sources | Secondary Sources |
|---|---|
| Face to face/Phone Interviews with market participants, such as: Manufactures; Distributors; End-users; Experts. Online Survey |
Government/International Organization Data: Annual Report/Presentation/Fact Book Internet Source Information Industry Association Data Free/Purchased Database Market Research Report Book/Journal/News |