Oncology-Based In vivo CRO Market Insights 2026, Analysis and Forecast to 2031
- Single User License (1 Users) $ 3,500
- Team License (2~5 Users) $ 4,500
- Corporate License (>5 Users) $ 5,500
The Histone Deacetylase (HDAC) inhibitors market is a specialized and high-growth segment of the epigenetic therapeutics industry, focused on small molecules that modify gene expression by inhibiting the enzymatic activity of histone deacetylases. This field has transitioned from basic research into a critical pillar of modern oncology, particularly for hematologic malignancies such as cutaneous T-cell lymphoma (CTCL), peripheral T-cell lymphoma (PTCL), and multiple myeloma. The market is defined by a strategic evolution from first-generation "pan-HDAC" inhibitors to "isoform-selective" agents designed to maximize therapeutic efficacy while mitigating systemic toxicities. Beyond oncology, the industry is increasingly exploring the role of HDAC inhibition in neurodegeneration, inflammatory diseases, and metabolic disorders, making it a versatile frontier for precision medicine. The global Histone Deacetylase Inhibitors market is estimated to reach a valuation of approximately USD 0.8–1.8 billion in 2025, with compound annual growth rates (CAGR) projected in the range of 4.0%–10.0% through 2030. This growth is sustained by rising global cancer incidences, a robust clinical pipeline of combination therapies, and increasing investment in epigenetic biomarker discovery to improve patient stratification.
Type Analysis and Market Segmentation
Class I HDAC Inhibitors Class I HDACs (encompassing isoforms 1, 2, 3, and 8) are primarily localized in the cell nucleus and play essential roles in cell cycle progression and survival. This segment is the most commercially mature, growing at an estimated CAGR of 4.5%–11.0%. Currently, the market trend is shifting toward "Class I Selective" inhibitors to reduce the adverse effects associated with broader inhibition. These agents are the standard of care in several refractory lymphoma indications and remain the primary focus of major pharmaceutical portfolios.
Class II HDAC Inhibitors Comprising Classes IIa (HDAC 4, 5, 7, 9) and IIb (HDAC 6, 10), this segment is expanding at an annual rate of 5.5%–13.0%. Class II inhibitors are unique for their ability to shuttle between the nucleus and the cytoplasm, regulating non-histone proteins. HDAC6-selective inhibitors, in particular, are garnering significant interest due to their potential in treating multiple myeloma and neurodegenerative conditions without the severe hematological toxicities often seen with Class I inhibition.
Class IV HDAC Inhibitors Focusing primarily on HDAC 11, this segment is growing at a rate of 3.5%–8.5%. While fewer approved agents exist in this category, HDAC 11 is recognized as a key regulator of immune cell function. Ongoing research is investigating its role in modulating the immune microenvironment, potentially opening new avenues for autoimmune and metabolic therapies.
Application Analysis and Market Trends
Pharmaceutical Companies and Research Institutes The R&D and pharmaceutical segment is expected to grow at 6.0%–14.0%. This reflects the massive capital allocation toward "combination therapy" trials, where HDAC inhibitors are paired with immune checkpoint inhibitors (PD-1/PD-L1) or proteasome inhibitors. The objective is to utilize HDACis to "prime" tumors, making them more susceptible to the immune system or other therapeutic agents.
Hospitals and Specialized Clinics Hospitals remain the dominant end-users for approved HDAC therapies, with growth projected at 4.0%–9.0%. The management of HDAC inhibitor regimens—which often require careful monitoring for side effects like thrombocytopenia or QTc prolongation—necessitates the advanced oncological infrastructure provided by hospital infusion centers and hematology departments.
Regional Market Distribution and Geographic Trends
North America: Projected growth of 4.0%–9.5%. The United States leads the global market, driven by a highly advanced biopharmaceutical sector, high healthcare R&D expenditure, and a favorable regulatory environment for orphan drug designations. The region benefits from early adoption of epigenetic screening and established reimbursement pathways for innovative oncology treatments.
Asia-Pacific: Estimated growth of 7.5%–14.5%. This is the fastest-growing region, propelled by expanding healthcare infrastructure in China and India. Chinese domestic innovators have successfully launched indigenous HDAC inhibitors, such as Chidamide, which are now being explored for global markets. Increased government support for biotechnology and a rising prevalence of target diseases in aging populations further bolster this region.
Europe: Projected growth of 3.5%–8.0%. Key markets including Germany, France, and the UK focus on rational drug design and precision oncology. European research institutions are at the forefront of identifying isoform-specific biomarkers, which is critical for the next generation of selective HDAC therapies.
Latin America and MEA: Estimated growth of 3.0%–9.0%. Growth in these regions is primarily driven by the expansion of private oncology networks and a growing awareness of personalized medicine in urban healthcare hubs.
Key Market Players and Competitive Landscape
The competitive landscape of the HDAC inhibitor market is a mix of global pharmaceutical giants and specialized biotech innovators.
Pharmaceutical Leaders: Bristol Myers Squibb and Merck & Co., Inc. maintain significant influence through their established portfolios and clinical trial leadership. Novartis AG remains a key player, particularly with its history in multiple myeloma treatments and ongoing efforts to develop oral formulations that enhance patient compliance.
Specialized Innovators: Shenzhen Chipscreen Biosciences Ltd. has become a notable global competitor with the development of the first subtype-selective HDAC inhibitor, Chidamide. Other specialized firms like 4SC AG, Celleron Therapeutics Ltd., and CrystalGenomics Inc. are actively advancing the pipeline with candidates targeting solid tumors and liver cancers.
Niche Biotech Players: Companies such as Chroma Therapeutics Ltd., Forum Pharmaceuticals Inc., and Curis, Inc. focus on "targeted delivery" and isoform-specific molecules. These players often drive the industry’s shift toward reducing the "off-target" effects that have historically limited the broader adoption of pan-HDAC inhibitors.
Industry Value Chain Analysis
The value chain for HDAC inhibitors is characterized by high technical barriers and a concentration of value in the late-stage clinical and commercialization phases.
Upstream Research and Drug Discovery: Value begins with high-throughput screening and computational modeling of the HDAC enzyme's catalytic site. Intellectual property is concentrated here, especially for molecules that demonstrate superior "zinc-binding" affinity and isoform selectivity.
Clinical Development and Biomarker Integration: This is the most critical stage of the chain. Companies add value by identifying biomarkers that predict which patients will respond to HDAC inhibition, thereby increasing the success rate of clinical trials and supporting a precision medicine narrative.
Active Pharmaceutical Ingredient (API) Synthesis: Manufacturing these molecules requires complex organic synthesis and high-purity standards. Value is added through the development of "Oral Bioavailability" technologies, allowing patients to take medications at home rather than requiring intravenous administration in a clinic.
Specialized Distribution and Pharmacy Networks: Due to the orphan drug status of many HDAC inhibitors, distribution is often handled through specialized oncology pharmacies. This ensures that the drug is accompanied by patient support programs and rigorous safety monitoring.
Clinical Adoption and Patient Care: The highest value is captured by healthcare providers who integrate these drugs into complex, multi-modal treatment plans. Effective management of the therapy's safety profile is essential for maintaining long-term commercial viability.
Market Opportunities and Challenges
Opportunities The most significant opportunity lies in "Non-Oncology Indications." There is burgeoning clinical evidence for the use of HDAC inhibitors in treating neurodegenerative diseases (like Alzheimer’s), where they may help restore synaptic plasticity. Furthermore, "Immuno-Oncology Combinations" represent a massive potential market; using HDAC inhibitors to turn "cold" tumors "hot" (more recognizable by the immune system) could expand their use from rare lymphomas to major solid tumors like lung or breast cancer. The shift toward "Selective Isoform Targeting" also offers the promise of chronic-use therapies with much safer toxicity profiles.
Challenges "Dose-Limiting Toxicities" remain a primary hurdle, as early-generation pan-HDAC inhibitors are often associated with fatigue, nausea, and hematological issues that can lead to high patient dropout rates. "High Development Costs" and the complexity of the epigenome also pose risks; a molecule may show promise in vitro but fail in vivo due to the dynamic nature of gene regulation. Additionally, competition from newer modalities such as CAR-T cell therapy and bispecific antibodies in the hematology space may restrict the growth of HDACis unless they are successfully integrated into combination regimens.
Chapter 1 Executive Summary
Chapter 2 Abbreviation and Acronyms
Chapter 3 Preface
3.1 Research Scope
3.2 Research Sources
3.2.1 Data Sources
3.2.2 Assumptions
3.3 Research Method
Chapter 4 Market Landscape
4.1 Market Overview
4.2 Classification/Types
4.3 Application/End Users
Chapter 5 Market Trend Analysis
5.1 Introduction
5.2 Drivers
5.3 Restraints
5.4 Opportunities
5.5 Threats
Chapter 6 Industry Chain Analysis
6.1 Upstream/Suppliers Analysis
6.2 Oncology-Based in vivo CRO Analysis
6.2.1 Technology Analysis
6.2.2 Cost Analysis
6.2.3 Market Channel Analysis
6.3 Downstream Buyers/End Users
Chapter 7 Latest Market Dynamics
7.1 Latest News
7.2 Merger and Acquisition
7.3 Planned/Future Project
7.4 Policy Dynamics
Chapter 8 Historical and Forecast Oncology-Based in vivo CRO Market in North America (2021-2031)
8.1 Oncology-Based in vivo CRO Market Size
8.2 Oncology-Based in vivo CRO Market by End Use
8.3 Competition by Players/Suppliers
8.4 Oncology-Based in vivo CRO Market Size by Type
8.5 Key Countries Analysis
8.5.1 United States
8.5.2 Canada
8.5.3 Mexico
Chapter 9 Historical and Forecast Oncology-Based in vivo CRO Market in South America (2021-2031)
9.1 Oncology-Based in vivo CRO Market Size
9.2 Oncology-Based in vivo CRO Market by End Use
9.3 Competition by Players/Suppliers
9.4 Oncology-Based in vivo CRO Market Size by Type
9.5 Key Countries Analysis
9.5.1 Brazil
9.5.2 Argentina
9.5.3 Chile
9.5.4 Peru
Chapter 10 Historical and Forecast Oncology-Based in vivo CRO Market in Asia & Pacific (2021-2031)
10.1 Oncology-Based in vivo CRO Market Size
10.2 Oncology-Based in vivo CRO Market by End Use
10.3 Competition by Players/Suppliers
10.4 Oncology-Based in vivo CRO Market Size by Type
10.5 Key Countries Analysis
10.5.1 China
10.5.2 India
10.5.3 Japan
10.5.4 South Korea
10.5.5 Southest Asia
10.5.6 Australia & New Zealand
Chapter 11 Historical and Forecast Oncology-Based in vivo CRO Market in Europe (2021-2031)
11.1 Oncology-Based in vivo CRO Market Size
11.2 Oncology-Based in vivo CRO Market by End Use
11.3 Competition by Players/Suppliers
11.4 Oncology-Based in vivo CRO Market Size by Type
11.5 Key Countries Analysis
11.5.1 Germany
11.5.2 France
11.5.3 United Kingdom
11.5.4 Italy
11.5.5 Spain
11.5.6 Belgium
11.5.7 Netherlands
11.5.8 Austria
11.5.9 Poland
11.5.10 North Europe
Chapter 12 Historical and Forecast Oncology-Based in vivo CRO Market in MEA (2021-2031)
12.1 Oncology-Based in vivo CRO Market Size
12.2 Oncology-Based in vivo CRO Market by End Use
12.3 Competition by Players/Suppliers
12.4 Oncology-Based in vivo CRO Market Size by Type
12.5 Key Countries Analysis
12.5.1 Egypt
12.5.2 Israel
12.5.3 South Africa
12.5.4 Gulf Cooperation Council Countries
12.5.5 Turkey
Chapter 13 Summary For Global Oncology-Based in vivo CRO Market (2021-2026)
13.1 Oncology-Based in vivo CRO Market Size
13.2 Oncology-Based in vivo CRO Market by End Use
13.3 Competition by Players/Suppliers
13.4 Oncology-Based in vivo CRO Market Size by Type
Chapter 14 Global Oncology-Based in vivo CRO Market Forecast (2026-2031)
14.1 Oncology-Based in vivo CRO Market Size Forecast
14.2 Oncology-Based in vivo CRO Application Forecast
14.3 Competition by Players/Suppliers
14.4 Oncology-Based in vivo CRO Type Forecast
Chapter 15 Analysis of Global Key Vendors
15.1 The Jackson Laboratory
15.1.1 Company Profile
15.1.2 Main Business and Oncology-Based in vivo CRO Information
15.1.3 SWOT Analysis of The Jackson Laboratory
15.1.4 The Jackson Laboratory Oncology-Based in vivo CRO Revenue, Cost and Gross Margin (2021-2026)
15.2 Crown Bioscience Inc.
15.2.1 Company Profile
15.2.2 Main Business and Oncology-Based in vivo CRO Information
15.2.3 SWOT Analysis of Crown Bioscience Inc.
15.2.4 Crown Bioscience Inc. Oncology-Based in vivo CRO Revenue, Cost and Gross Margin (2021-2026)
15.3 Taconic Biosciences Inc.
15.3.1 Company Profile
15.3.2 Main Business and Oncology-Based in vivo CRO Information
15.3.3 SWOT Analysis of Taconic Biosciences Inc.
15.3.4 Taconic Biosciences Inc. Oncology-Based in vivo CRO Revenue, Cost and Gross Margin (2021-2026)
15.4 Charles River Laboratories International Inc.
15.4.1 Company Profile
15.4.2 Main Business and Oncology-Based in vivo CRO Information
15.4.3 SWOT Analysis of Charles River Laboratories International Inc.
15.4.4 Charles River Laboratories International Inc. Oncology-Based in vivo CRO Revenue, Cost and Gross Margin (2021-2026)
15.5 LabCorp
15.5.1 Company Profile
15.5.2 Main Business and Oncology-Based in vivo CRO Information
15.5.3 SWOT Analysis of LabCorp
15.5.4 LabCorp Oncology-Based in vivo CRO Revenue, Cost and Gross Margin (2021-2026)
15.6 Eurofins Scientific
15.6.1 Company Profile
15.6.2 Main Business and Oncology-Based in vivo CRO Information
15.6.3 SWOT Analysis of Eurofins Scientific
15.6.4 Eurofins Scientific Oncology-Based in vivo CRO Revenue, Cost and Gross Margin (2021-2026)
15.7 Harbour BioMed
15.7.1 Company Profile
15.7.2 Main Business and Oncology-Based in vivo CRO Information
15.7.3 SWOT Analysis of Harbour BioMed
15.7.4 Harbour BioMed Oncology-Based in vivo CRO Revenue, Cost and Gross Margin (2021-2026)
Please ask for sample pages for full companies list
Table Research Scope of Oncology-Based In vivo CRO Report
Table Data Sources of Oncology-Based In vivo CRO Report
Table Major Assumptions of Oncology-Based In vivo CRO Report
Table Oncology-Based In vivo CRO Classification
Table Oncology-Based In vivo CRO Applications
Table Drivers of Oncology-Based In vivo CRO Market
Table Restraints of Oncology-Based In vivo CRO Market
Table Opportunities of Oncology-Based In vivo CRO Market
Table Threats of Oncology-Based In vivo CRO Market
Table Raw Materials Suppliers
Table Different Production Methods of Oncology-Based In vivo CRO
Table Cost Structure Analysis of Oncology-Based In vivo CRO
Table Key End Users
Table Latest News of Oncology-Based In vivo CRO Market
Table Merger and Acquisition
Table Planned/Future Project of Oncology-Based In vivo CRO Market
Table Policy of Oncology-Based In vivo CRO Market
Table 2021-2031 North America Oncology-Based In vivo CRO Market Size
Table 2021-2031 North America Oncology-Based In vivo CRO Market Size by Application
Table 2021-2026 North America Oncology-Based In vivo CRO Key Players Revenue
Table 2021-2026 North America Oncology-Based In vivo CRO Key Players Market Share
Table 2021-2031 North America Oncology-Based In vivo CRO Market Size by Type
Table 2021-2031 United States Oncology-Based In vivo CRO Market Size
Table 2021-2031 Canada Oncology-Based In vivo CRO Market Size
Table 2021-2031 Mexico Oncology-Based In vivo CRO Market Size
Table 2021-2031 South America Oncology-Based In vivo CRO Market Size
Table 2021-2031 South America Oncology-Based In vivo CRO Market Size by Application
Table 2021-2026 South America Oncology-Based In vivo CRO Key Players Revenue
Table 2021-2026 South America Oncology-Based In vivo CRO Key Players Market Share
Table 2021-2031 South America Oncology-Based In vivo CRO Market Size by Type
Table 2021-2031 Brazil Oncology-Based In vivo CRO Market Size
Table 2021-2031 Argentina Oncology-Based In vivo CRO Market Size
Table 2021-2031 Chile Oncology-Based In vivo CRO Market Size
Table 2021-2031 Peru Oncology-Based In vivo CRO Market Size
Table 2021-2031 Asia & Pacific Oncology-Based In vivo CRO Market Size
Table 2021-2031 Asia & Pacific Oncology-Based In vivo CRO Market Size by Application
Table 2021-2026 Asia & Pacific Oncology-Based In vivo CRO Key Players Revenue
Table 2021-2026 Asia & Pacific Oncology-Based In vivo CRO Key Players Market Share
Table 2021-2031 Asia & Pacific Oncology-Based In vivo CRO Market Size by Type
Table 2021-2031 China Oncology-Based In vivo CRO Market Size
Table 2021-2031 India Oncology-Based In vivo CRO Market Size
Table 2021-2031 Japan Oncology-Based In vivo CRO Market Size
Table 2021-2031 South Korea Oncology-Based In vivo CRO Market Size
Table 2021-2031 Southeast Asia Oncology-Based In vivo CRO Market Size
Table 2021-2031 Australia & New ZealandOncology-Based In vivo CRO Market Size
Table 2021-2031 Europe Oncology-Based In vivo CRO Market Size
Table 2021-2031 Europe Oncology-Based In vivo CRO Market Size by Application
Table 2021-2026 Europe Oncology-Based In vivo CRO Key Players Revenue
Table 2021-2026 Europe Oncology-Based In vivo CRO Key Players Market Share
Table 2021-2031 Europe Oncology-Based In vivo CRO Market Size by Type
Table 2021-2031 Germany Oncology-Based In vivo CRO Market Size
Table 2021-2031 France Oncology-Based In vivo CRO Market Size
Table 2021-2031 United Kingdom Oncology-Based In vivo CRO Market Size
Table 2021-2031 Italy Oncology-Based In vivo CRO Market Size
Table 2021-2031 Spain Oncology-Based In vivo CRO Market Size
Table 2021-2031 Belgium Oncology-Based In vivo CRO Market Size
Table 2021-2031 Netherlands Oncology-Based In vivo CRO Market Size
Table 2021-2031 Austria Oncology-Based In vivo CRO Market Size
Table 2021-2031 Poland Oncology-Based In vivo CRO Market Size
Table 2021-2031 North Europe Oncology-Based In vivo CRO Market Size
Table 2021-2031 MEA Oncology-Based In vivo CRO Market Size
Table 2021-2031 MEA Oncology-Based In vivo CRO Market Size by Application
Table 2021-2026 MEA Oncology-Based In vivo CRO Key Players Revenue
Table 2021-2026 MEA Oncology-Based In vivo CRO Key Players Market Share
Table 2021-2031 MEA Oncology-Based In vivo CRO Market Size by Type
Table 2021-2031 Egypt Oncology-Based In vivo CRO Market Size
Table 2021-2031 Israel Oncology-Based In vivo CRO Market Size
Table 2021-2031 South Africa Oncology-Based In vivo CRO Market Size
Table 2021-2031 Gulf Cooperation Council Countries Oncology-Based In vivo CRO Market Size
Table 2021-2031 Turkey Oncology-Based In vivo CRO Market Size
Table 2021-2026 Global Oncology-Based In vivo CRO Market Size by Region
Table 2021-2026 Global Oncology-Based In vivo CRO Market Size Share by Region
Table 2021-2026 Global Oncology-Based In vivo CRO Market Size by Application
Table 2021-2026 Global Oncology-Based In vivo CRO Market Share by Application
Table 2021-2026 Global Oncology-Based In vivo CRO Key Vendors Revenue
Table 2021-2026 Global Oncology-Based In vivo CRO Key Vendors Market Share
Table 2021-2026 Global Oncology-Based In vivo CRO Market Size by Type
Table 2021-2026 Global Oncology-Based In vivo CRO Market Share by Type
Table 2026-2031 Global Oncology-Based In vivo CRO Market Size by Region
Table 2026-2031 Global Oncology-Based In vivo CRO Market Size Share by Region
Table 2026-2031 Global Oncology-Based In vivo CRO Market Size by Application
Table 2026-2031 Global Oncology-Based In vivo CRO Market Share by Application
Table 2026-2031 Global Oncology-Based In vivo CRO Key Vendors Revenue
Table 2026-2031 Global Oncology-Based In vivo CRO Key Vendors Market Share
Table 2026-2031 Global Oncology-Based In vivo CRO Market Size by Type
Table 2026-2031 Oncology-Based In vivo CRO Global Market Share by Type
Figure Market Size Estimated Method
Figure Major Forecasting Factors
Figure Oncology-Based In vivo CRO Picture
Figure 2021-2031 North America Oncology-Based In vivo CRO Market Size and CAGR
Figure 2021-2031 South America Oncology-Based In vivo CRO Market Size and CAGR
Figure 2021-2031 Asia & Pacific Oncology-Based In vivo CRO Market Size and CAGR
Figure 2021-2031 Europe Oncology-Based In vivo CRO Market Size and CAGR
Figure 2021-2031 MEA Oncology-Based In vivo CRO Market Size and CAGR
Figure 2021-2026 Global Oncology-Based In vivo CRO Market Size and Growth Rate
Figure 2026-2031 Global Oncology-Based In vivo CRO Market Size and Growth Rate
Research Methodology
- Market Estimated Methodology:
Bottom-up & top-down approach, supply & demand approach are the most important method which is used by HDIN Research to estimate the market size.
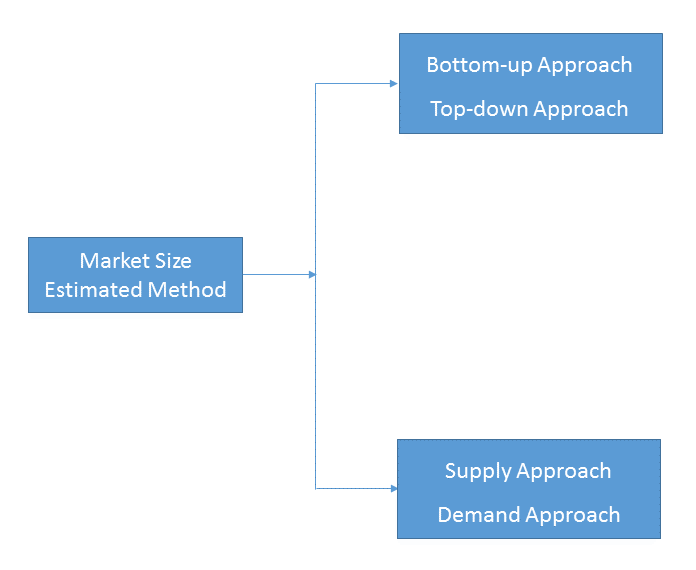
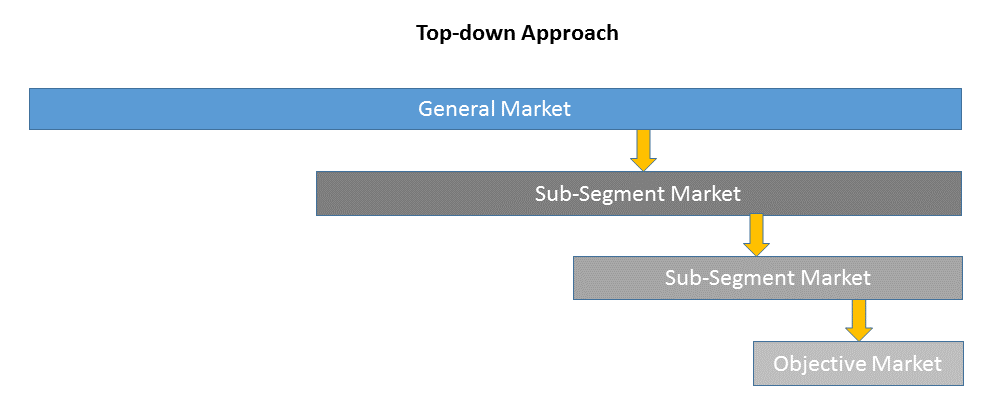
1)Top-down & Bottom-up Approach
Top-down approach uses a general market size figure and determines the percentage that the objective market represents.
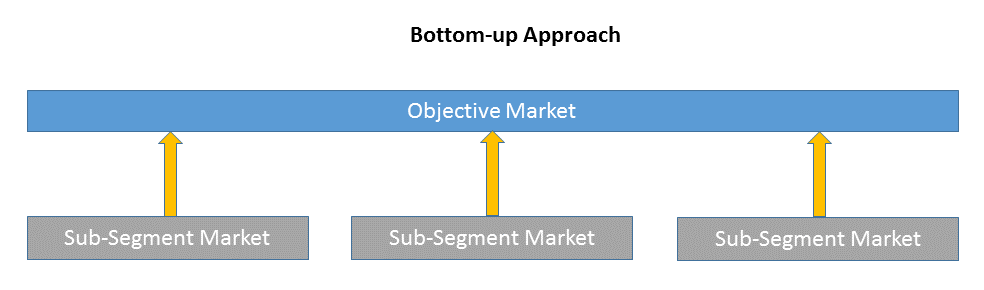
Bottom-up approach size the objective market by collecting the sub-segment information.
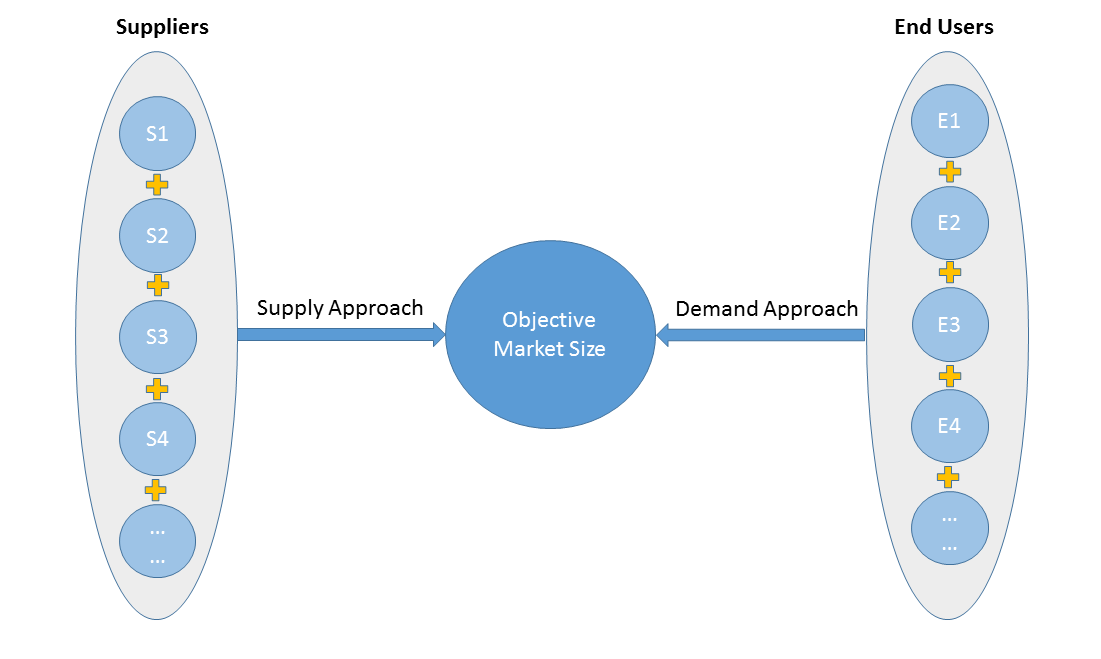
2)Supply & Demand Approach
Supply approach is based on assessments of the size of each competitor supplying the objective market.
Demand approach combine end-user data within a market to estimate the objective market size. It is sometimes referred to as bottom-up approach.
- Forecasting Methodology
- Numerous factors impacting the market trend are considered for forecast model:
- New technology and application in the future;
- New project planned/under contraction;
- Global and regional underlying economic growth;
- Threatens of substitute products;
- Industry expert opinion;
- Policy and Society implication.
- Analysis Tools
1)PEST Analysis
PEST Analysis is a simple and widely used tool that helps our client analyze the Political, Economic, Socio-Cultural, and Technological changes in their business environment.
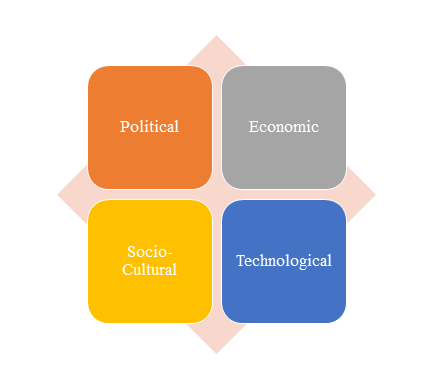
- Benefits of a PEST analysis:
- It helps you to spot business opportunities, and it gives you advanced warning of significant threats.
- It reveals the direction of change within your business environment. This helps you shape what you’re doing, so that you work with change, rather than against it.
- It helps you avoid starting projects that are likely to fail, for reasons beyond your control.
- It can help you break free of unconscious assumptions when you enter a new country, region, or market; because it helps you develop an objective view of this new environment.
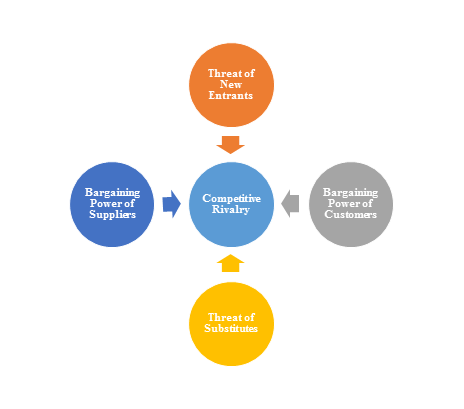
2)Porter’s Five Force Model Analysis
The Porter’s Five Force Model is a tool that can be used to analyze the opportunities and overall competitive advantage. The five forces that can assist in determining the competitive intensity and potential attractiveness within a specific area.
- Threat of New Entrants: Profitable industries that yield high returns will attract new firms.
- Threat of Substitutes: A substitute product uses a different technology to try to solve the same economic need.
- Bargaining Power of Customers: the ability of customers to put the firm under pressure, which also affects the customer's sensitivity to price changes.
- Bargaining Power of Suppliers: Suppliers of raw materials, components, labor, and services (such as expertise) to the firm can be a source of power over the firm when there are few substitutes.
- Competitive Rivalry: For most industries the intensity of competitive rivalry is the major determinant of the competitiveness of the industry.
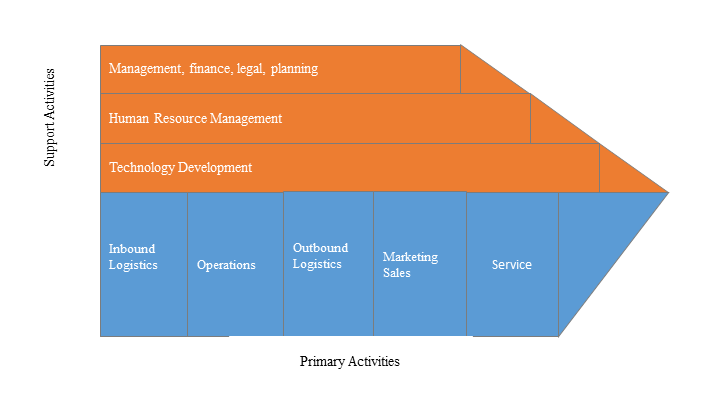
3)Value Chain Analysis
Value chain analysis is a tool to identify activities, within and around the firm and relating these activities to an assessment of competitive strength. Value chain can be analyzed by primary activities and supportive activities. Primary activities include: inbound logistics, operations, outbound logistics, marketing & sales, service. Support activities include: technology development, human resource management, management, finance, legal, planning.
4)SWOT Analysis
SWOT analysis is a tool used to evaluate a company's competitive position by identifying its strengths, weaknesses, opportunities and threats. The strengths and weakness is the inner factor; the opportunities and threats are the external factor. By analyzing the inner and external factors, the analysis can provide the detail information of the position of a player and the characteristics of the industry.
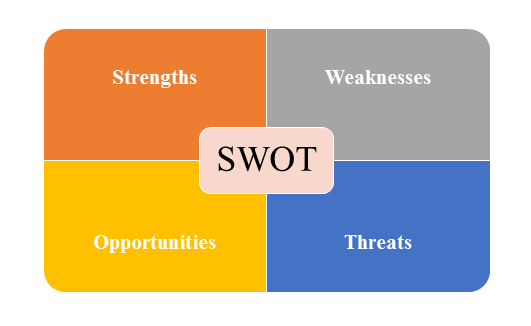
- Strengths describe what the player excels at and separates it from the competition
- Weaknesses stop the player from performing at its optimum level.
- Opportunities refer to favorable external factors that the player can use to give it a competitive advantage.
- Threats refer to factors that have the potential to harm the player.
- Data Sources
| Primary Sources | Secondary Sources |
|---|---|
| Face to face/Phone Interviews with market participants, such as: Manufactures; Distributors; End-users; Experts. Online Survey |
Government/International Organization Data: Annual Report/Presentation/Fact Book Internet Source Information Industry Association Data Free/Purchased Database Market Research Report Book/Journal/News |