Acromegaly Treatment Drugs Market Insights 2026, Analysis and Forecast to 2031
- Single User License (1 Users) $ 3,500
- Team License (2~5 Users) $ 4,500
- Corporate License (>5 Users) $ 5,500
Industry Overview and Disease Context
Acromegaly is a rare, chronic, and potentially life-threatening hormonal disorder characterized by the excessive production of growth hormone (GH) and the subsequent elevation of insulin-like growth factor-1 (IGF-1). In more than 95% of cases, the condition is caused by a benign tumor (adenoma) in the pituitary gland. If left untreated, the chronic elevation of these hormones leads to significant multisystemic complications, including cardiovascular disease, diabetes, respiratory failure, and progressive bone and joint disfigurement. Due to the insidious nature of the disease, diagnosis is often delayed by five to ten years, making medical intervention a critical pillar of patient management.
The acromegaly treatment drugs market serves patients for whom surgical resection of the pituitary tumor is either contraindicated, incomplete, or unsuccessful. Medical therapy has evolved from simple symptom management to a sophisticated pharmacological landscape aimed at biochemical control (normalization of GH and IGF-1 levels) and tumor shrinkage. The market is defined by high-value specialty medicines, orphan drug designations, and a recent shift toward patient-centric delivery systems, such as long-acting injectables and innovative oral formulations.
Market Scale and Growth Projections
The global acromegaly treatment drugs market is positioned within the broader orphan drug and endocrine disorder segments. It is characterized by high barriers to entry, high treatment costs, and a highly specialized prescribing base consisting mainly of endocrinologists and neurosurgeons.
* 2026 Estimated Market Size: The global market valuation is projected to reach between 2.5 billion USD and 3.8 billion USD by 2026. This valuation reflects the steady uptake of second-generation somatostatin analogues and the introduction of novel oral therapies.
* Compound Annual Growth Rate (CAGR): From 2026 through 2031, the market is anticipated to expand at a CAGR of 6.5% to 9.5%.
The growth of this market is primarily driven by improvements in diagnostic techniques, an increasing prevalence of diagnosed cases due to better disease awareness, and the premium pricing of newly approved therapies. Furthermore, the transition of patients from first-generation injectables to more convenient and effective second-generation or oral treatments is a significant value driver.
Product Type Analysis and Therapeutic Landscape
The market is categorized into three primary pharmacological classes, each addressing different points in the disease’s biological pathway.
● Somatostatin Analogues (SRLs)
Somatostatin Receptor Ligands (SRLs) are the cornerstone of medical therapy for acromegaly. They work by mimicking the natural inhibitory hormone somatostatin, binding to specific receptors (primarily SSTR2 and SSTR5) on the pituitary tumor to inhibit GH secretion.
* Key Products and Developments: This segment includes established long-acting injectables and the latest oral innovations. Somatuline® Depot (lanreotide) by Ipsen Biopharmaceuticals and Signifor® LAR (pasireotide), managed by Recordati and Novartis, are leading injectable options. Signifor® LAR is particularly noted for its high affinity for SSTR5, making it effective for patients resistant to other SRLs.
* The Oral Revolution: A significant shift is occurring with the introduction of MYCAPSSA® (octreotide capsules) by Chiesi Farmaceutici, the first oral SRL. Additionally, Crinetics Pharmaceuticals is poised to disrupt the market with PALSONIFY™ (paltusotine), a non-peptide, once-daily oral selective somatostatin receptor ligand. The shift from painful, healthcare-provider-administered injections to oral capsules is a major trend improving patient compliance and quality of life.
● Growth Hormone Receptor Antagonists
When patients do not achieve biochemical control with SRLs, growth hormone receptor antagonists are employed. Unlike SRLs, which inhibit GH production, these drugs block the action of GH at the cellular level, effectively preventing the production of IGF-1.
* Lead Product: SOMAVERT® (pegvisomant) by Pfizer remains the gold standard in this class. It is highly effective in normalizing IGF-1 levels even in treatment-resistant patients. Although it does not shrink the pituitary tumor, its peripheral action makes it a vital component of the therapeutic arsenal.
● Dopamine Agonists
Dopamine agonists are often used as adjunctive therapy or for patients with mild disease or tumors that secrete both GH and prolactin.
* Lead Product: Dostinex (cabergoline) by Pfizer is frequently used off-label or in specific combined regimens. While generally less potent than SRLs, dopamine agonists offer the advantage of oral administration and lower costs, though they are rarely used as monotherapy for severe cases.
Key Market Players and Strategic Profiles
The competitive landscape is dominated by large pharmaceutical companies and specialized biotechs that focus on rare endocrine disorders.
* Novartis & Recordati Rare Diseases Inc.: Novartis was a pioneer in the acromegaly space with the development of pasireotide. Through strategic agreements, Recordati Rare Diseases now manages the commercialization of Signifor® LAR, focusing on its use for patients who remain uncontrolled on first-generation SRLs. Their involvement ensures the continued availability of high-potency treatments for complex cases.
* Ipsen Biopharmaceuticals Inc.: Ipsen is a dominant player through Somatuline® Depot. Their focus has been on providing long-acting formulations that reduce the burden of frequent injections. Ipsen continues to invest in life-cycle management and patient support programs to maintain its significant market share against emerging oral competitors.
* Pfizer: Pfizer holds a unique position with SOMAVERT®, the only GH receptor antagonist on the market. By providing a secondary mechanism of action, Pfizer captures the segment of the patient population that is refractory to SRLs. Their legacy dopamine agonist, Dostinex, also provides a low-cost entry point for adjunctive therapy.
* Chiesi Farmaceutici S.p.A.: Chiesi has positioned itself as an innovator in patient-centric care following the acquisition of Amryt Pharma, which brought MYCAPSSA® into its portfolio. Chiesi is leading the "oralization" of the acromegaly market, targeting patients who suffer from "injection fatigue" or sub-optimal control on injectables.
* Crinetics Pharmaceuticals Inc.: Crinetics is a critical "disruptor" in this space. Their lead candidate, paltusotine (PALSONIFY™), represents a new generation of oral non-peptide therapies. Crinetics focuses on a selective mechanism that offers the potential for once-daily dosing with a safety profile that could challenge the current injectable standards.
* Crinetics Pharmaceuticals Inc. (Pasireotide involvement): While pasireotide is traditionally associated with Novartis/Recordati, Crinetics has been involved in developing next-generation endocrine therapies that complement or enhance the existing pasireotide clinical landscape through advanced research and potential combination perspectives.
Regional Market Analysis and Trends
● North America
* Estimated Market Share: 40% - 45%
* Trends: The United States represents the largest individual market due to high healthcare expenditure, early adoption of novel orphan drugs, and a well-established network of Pituitary Centers of Excellence. The shift toward oral therapies is most pronounced here, driven by patient demand and favorable reimbursement for specialty oral medicines.
* Diagnosis & Awareness: High rates of IGF-1 screening and the presence of strong patient advocacy groups (such as the Pituitary Network Association) contribute to a higher diagnosed prevalence compared to other regions.
● Europe
* Estimated Market Share: 25% - 30%
* Trends: Markets in Germany, France, and the UK are characterized by strong regulatory support for orphan drugs (EMA) but rigorous health technology assessments (HTA).
* Reimbursement Dynamics: National health systems in Europe are increasingly focusing on the cost-effectiveness of second-line therapies. While injectables remain the standard, the introduction of oral alternatives is being carefully evaluated for their potential to reduce the overall "cost of care" by eliminating the need for professional administration in clinics.
● Asia-Pacific (APAC)
* Estimated Market Share: 15% - 20%
* Trends: APAC is expected to be the fastest-growing region through 2031. This is driven by improvements in healthcare infrastructure in China and India and increasing awareness among clinicians.
* Market Entry: Many global players are expanding their presence in Japan and China. The rising middle class and expansion of insurance coverage for rare diseases in China are unlocking significant patient populations that were previously under-treated.
● South America and Middle East & Africa (MEA)
* Estimated Market Share: 5% - 10% (Combined)
* Trends: These regions represent significant unmet needs. In the MEA region, particularly in the GCC countries, there is a high focus on providing world-class care for rare diseases. In South America, Brazil is a key market where government-funded access to high-cost medicines is a critical factor for market penetration.
Value Chain and Industry Structure
The value chain for acromegaly drugs is complex and necessitates specialized capabilities at every stage.
1. Research & Development (Endocrine Focus): R&D is the most critical phase. Developing molecules that can survive the digestive system (for oral drugs) or maintain sustained release (for injectables) requires high-level chemical and biological engineering.
2. Manufacturing (Specialized API & Formulation): The production of somatostatin analogues involves complex peptide synthesis. For products like Somatuline® Depot, the manufacturing of the specialized pre-filled syringe and the viscous gel formulation is a high-barrier technical requirement.
3. Regulatory Approval & Orphan Designation: Companies leverage "Orphan Drug" status to obtain tax credits, R&D grants, and extended market exclusivity (typically 7 years in the US and 10 in the EU). This is vital to offset the high costs of developing drugs for a small patient population.
4. Specialty Pharmacy & Cold Chain Distribution: Many acromegaly drugs require temperature-controlled shipping. Specialty pharmacies play a crucial role in patient education, ensuring adherence, and navigating insurance authorizations.
5. Clinical Integration: The end of the chain involves multidisciplinary teams at specialized pituitary centers. Success in this market depends heavily on "Medical Science Liaisons" (MSLs) building relationships with key opinion leaders (KOLs) in endocrinology.
Market Opportunities
1. The Transition to Oral Non-Peptide Therapies
The primary opportunity in the acromegaly market is the replacement of injectable therapies with oral alternatives. Patients frequently report "injection site pain," "injection site reactions," and "breakthrough symptoms" toward the end of their 28-day injection cycle. Non-peptide orals like paltusotine offer a more stable steady-state concentration and significantly higher patient convenience, which is expected to capture a large share of the "well-controlled" injectable market.
2. Digital Health and Integrated Care Models
There is a growing opportunity for "Drug-Plus" models. This involves the use of wearable sensors and digital apps to monitor symptom severity and IGF-1 levels. Integrated care models that connect patients with endocrine nurses can improve adherence and provide pharmaceutical companies with real-world evidence (RWE) to support reimbursement negotiations.
3. Early Diagnosis and Biomarker Discovery
Advancements in medical imaging and the discovery of more sensitive biomarkers for GH/IGF-1 activity allow for earlier intervention. As primary care physicians become better educated on the subtle early signs of acromegaly, the pool of treated patients will expand, particularly in emerging markets.
4. Expansion into Pediatric and GH-Related Conditions
While acromegaly primarily affects adults, the underlying technology of GH suppression has applications in other conditions, such as gigantism in children or certain types of endocrine-driven tumors. Expanding the labels of existing drugs offers a pathway for low-risk revenue growth.
Market Challenges
1. High Cost of Therapy and Reimbursement Hurdles
Acromegaly drugs are among the most expensive specialty medications. In an era of tightening healthcare budgets, payers are demanding more evidence of "value-based" outcomes. Manufacturers face significant hurdles in justifying premium prices, especially when first-generation generics or biosimilars (though currently limited) might enter the market.
2. Diagnosis Delays and Low Prevalence
Acromegaly is an ultra-rare disease with a global prevalence of roughly 60 to 86 cases per million people. The small patient pool limits the total addressable market. Furthermore, because the symptoms (hand/foot enlargement, joint pain) are often attributed to aging or common orthopedic issues, the average time to diagnosis remains excessively high, delaying the initiation of pharmaceutical therapy.
3. Treatment Resistance and Heterogeneity
A significant portion of patients (up to 30-40%) does not achieve full biochemical control on current first-line somatostatin analogues. The heterogeneous nature of pituitary tumors means that some lack the specific receptors (SSTR2) targeted by most drugs. Developing "universal" ligands or effectively managing "refractory" patients requires ongoing high-cost clinical trials.
4. Competitive Dynamics and Patent Cliffs
The entry of oral therapies poses a threat to the established injectable franchises. As older products face patent expirations, the entry of biosimilars for somatostatin analogues could lead to price erosion in the injectable segment, forcing incumbents to accelerate their shift toward more innovative, protected delivery systems.
5. Stringent Regulatory Requirements
The FDA and EMA have high bars for safety, particularly regarding the glycemic impact of certain treatments. For instance, pasireotide is known to affect glucose metabolism, requiring careful monitoring. Navigating these safety concerns while maintaining efficacy is a constant challenge for drug developers in the acromegaly space.
1.1 Study Scope 1
1.2 Research Methodology 2
1.2.1 Data Sources 2
1.2.2 Assumptions 3
1.3 Abbreviations and Acronyms 5
Chapter 2 Global Acromegaly Treatment Drugs Market Assessment 7
2.1 Market Overview and Definition 7
2.2 Global Market Size and Growth Trajectory (2021-2031) 8
2.3 Key Market Drivers and Opportunities 9
2.4 Major Restraints and Challenges 11
2.5 Industry Trends (Shift to Oral Somatostatin Analogues) 12
Chapter 3 Value Chain and R&D Analysis 13
3.1 Industry Value Chain Analysis 13
3.2 Drug Development and Clinical Trials Landscape 14
3.3 Manufacturing and Supply Chain Complexity 15
3.4 Patent Landscape and Expiry Analysis 16
Chapter 4 Market Segmentation by Type 17
4.1 Global Market Share by Type (2021-2031) 17
4.2 Somatostatin Analogues (SRLs) 18
4.2.1 First-Generation SRLs 18
4.2.2 Second-Generation SRLs 19
4.3 Growth Hormone Receptor Antagonists 20
4.4 Dopamine Agonists 21
4.5 Combination Therapies 22
Chapter 5 Market Segmentation by Distribution Channel 23
5.1 Global Market Share by Channel (2021-2031) 23
5.2 Hospital Pharmacies 24
5.3 Retail Pharmacies 25
5.4 Online Pharmacies and Specialty Clinics 26
Chapter 6 Global Market Analysis by Region 28
6.1 Global Market Share by Region (2021-2031) 28
6.2 North America Market Status and Prospect 29
6.3 Europe Market Status and Prospect 30
6.4 Asia-Pacific Market Status and Prospect 31
6.5 Latin America, Middle East & Africa Market Status and Prospect 32
Chapter 7 North America Acromegaly Treatment Drugs Market 33
7.1 North America Market Size and Forecast 33
7.2 United States 34
7.3 Canada 36
7.4 Mexico 37
Chapter 8 Europe Acromegaly Treatment Drugs Market 39
8.1 Europe Market Size and Forecast 39
8.2 Germany 40
8.3 France 41
8.4 United Kingdom 42
8.5 Italy 43
8.6 Rest of Europe 44
Chapter 9 Asia-Pacific Acromegaly Treatment Drugs Market 45
9.1 Asia-Pacific Market Size and Forecast 45
9.2 China 46
9.3 Japan 47
9.4 India 47
9.5 South Korea 48
9.6 Taiwan (China) 48
9.7 Southeast Asia 49
Chapter 10 Latin America, Middle East & Africa Acromegaly Treatment Drugs Market 50
10.1 LAMEA Market Size and Forecast 50
10.2 Brazil 51
10.3 Saudi Arabia 52
10.4 South Africa 53
Chapter 11 Competitive Landscape 54
11.1 Global Market Share Analysis by Player (2026) 54
11.2 Market Concentration Rate (CR3, CR5) 55
11.3 Mergers, Acquisitions, and Strategic Partnerships 56
11.4 Pipeline Analysis and Emerging Competitors 58
Chapter 12 Company Profiles 60
12.1 Novartis 60
12.1.1 Company Overview 60
12.1.2 SWOT Analysis 60
12.1.3 Product Portfolio and R&D Strategy 61
12.1.4 Novartis Acromegaly Treatment Drugs Financial Analysis 62
12.2 Ipsen Biopharmaceuticals Inc. 64
12.2.1 Company Overview 64
12.2.2 SWOT Analysis 64
12.2.3 Product Portfolio and Marketing Strategy 65
12.2.4 Ipsen Biopharmaceuticals Inc. Acromegaly Treatment Drugs Financial Analysis 66
12.3 Pfizer 68
12.3.1 Company Overview 68
12.3.2 SWOT Analysis 68
12.3.3 Product Portfolio and Regional Presence 69
12.3.4 Pfizer Acromegaly Treatment Drugs Financial Analysis 70
12.4 Crinetics Pharmaceuticals Inc 72
12.4.1 Company Overview 72
12.4.2 SWOT Analysis 72
12.4.3 Pipeline Development (Paltusotine) and Commercialization 73
12.4.4 Crinetics Pharmaceuticals Inc Acromegaly Treatment Drugs Financial Analysis 74
12.5 Recordati Rare Diseases Inc. 76
12.5.1 Company Overview 76
12.5.2 SWOT Analysis 76
12.5.3 Niche Market Strategy 77
12.5.4 Recordati Rare Diseases Inc. Acromegaly Treatment Drugs Financial Analysis 78
12.6 Chiesi Farmaceutici S.p.A. 80
12.6.1 Company Overview 80
12.6.2 SWOT Analysis 80
12.6.3 Oral Formulation Strategy (Mycapssa) 81
12.6.4 Chiesi Farmaceutici S.p.A. Acromegaly Treatment Drugs Financial Analysis 82
Chapter 13 Market Forecast (2027-2031) 84
13.1 Global Revenue Forecast 84
13.2 Forecast by Type 85
13.3 Forecast by Region 86
Chapter 14 Regulatory and Reimbursement Landscape 87
14.1 Orphan Drug Designation Policies 87
14.2 Pricing and Reimbursement Challenges 88
Chapter 15 Conclusion and Recommendations 89
15.1 Key Findings 89
15.2 Strategic Recommendations 89
Table 1. Data Sources and Research Validation 2
Table 2. List of Abbreviations 5
Table 3. Global Acromegaly Treatment Drugs Market Revenue (USD Million) by Type, 2021-2026 17
Table 4. Global Acromegaly Treatment Drugs Market Revenue (USD Million) by Type, Forecast 2027-2031 17
Table 5. Global Acromegaly Treatment Drugs Market Revenue (USD Million) by Distribution Channel, 2021-2026 23
Table 6. Global Acromegaly Treatment Drugs Market Revenue (USD Million) by Distribution Channel, Forecast 2027-2031 24
Table 7. Global Acromegaly Treatment Drugs Market Revenue (USD Million) by Region, 2021-2026 28
Table 8. Global Acromegaly Treatment Drugs Market Revenue (USD Million) by Region, Forecast 2027-2031 29
Table 9. North America Acromegaly Treatment Drugs Market Revenue by Country, 2021-2031 33
Table 10. Europe Acromegaly Treatment Drugs Market Revenue by Country, 2021-2031 39
Table 11. Asia-Pacific Acromegaly Treatment Drugs Market Revenue by Country, 2021-2031 45
Table 12. Key Mergers, Acquisitions, and Partnerships in Acromegaly Space 56
Table 13. Novartis Acromegaly Treatment Drugs Revenue, Cost and Gross Profit Margin (2021-2026) 62
Table 14. Ipsen Biopharmaceuticals Inc. Acromegaly Treatment Drugs Revenue, Cost and Gross Profit Margin (2021-2026) 66
Table 15. Pfizer Acromegaly Treatment Drugs Revenue, Cost and Gross Profit Margin (2021-2026) 70
Table 16. Crinetics Pharmaceuticals Inc Acromegaly Treatment Drugs Revenue, Cost and Gross Profit Margin (2021-2026) 74
Table 17. Recordati Rare Diseases Inc. Acromegaly Treatment Drugs Revenue, Cost and Gross Profit Margin (2021-2026) 78
Table 18. Chiesi Farmaceutici S.p.A. Acromegaly Treatment Drugs Revenue, Cost and Gross Profit Margin (2021-2026) 82
Table 19. Regulatory Approvals and Pipeline Candidates 87
List of Figures
Figure 1. Acromegaly Treatment Drugs Research Methodology 2
Figure 2. Global Acromegaly Treatment Drugs Market Revenue (USD Million), 2021-2031 8
Figure 3. Global Acromegaly Treatment Drugs Market Share by Type, 2026 17
Figure 4. Global Somatostatin Analogues (SRLs) Market Revenue (USD Million), 2021-2031 18
Figure 5. Global Growth Hormone Receptor Antagonists Market Revenue (USD Million), 2021-2031 20
Figure 6. Global Dopamine Agonists Market Revenue (USD Million), 2021-2031 21
Figure 7. Global Acromegaly Treatment Drugs Market Share by Distribution Channel, 2026 23
Figure 8. Global Acromegaly Treatment Drugs Market Share by Region, 2026 28
Figure 9. North America Acromegaly Treatment Drugs Market Revenue (USD Million), 2021-2031 33
Figure 10. United States Acromegaly Treatment Drugs Market Revenue (USD Million), 2021-2031 35
Figure 11. Europe Acromegaly Treatment Drugs Market Revenue (USD Million), 2021-2031 39
Figure 12. Asia-Pacific Acromegaly Treatment Drugs Market Revenue (USD Million), 2021-2031 45
Figure 13. LAMEA Acromegaly Treatment Drugs Market Revenue (USD Million), 2021-2031 50
Figure 14. Global Acromegaly Treatment Drugs Market Share by Key Players, 2026 54
Figure 15. Novartis Acromegaly Treatment Drugs Market Share (2021-2026) 63
Figure 16. Ipsen Biopharmaceuticals Inc. Acromegaly Treatment Drugs Market Share (2021-2026) 67
Figure 17. Pfizer Acromegaly Treatment Drugs Market Share (2021-2026) 71
Figure 18. Crinetics Pharmaceuticals Inc Acromegaly Treatment Drugs Market Share (2021-2026) 75
Figure 19. Recordati Rare Diseases Inc. Acromegaly Treatment Drugs Market Share (2021-2026) 79
Figure 20. Chiesi Farmaceutici S.p.A. Acromegaly Treatment Drugs Market Share (2021-2026) 83
Figure 21. Global Acromegaly Treatment Drugs Revenue Forecast (USD Million), 2027-2031 84
Research Methodology
- Market Estimated Methodology:
Bottom-up & top-down approach, supply & demand approach are the most important method which is used by HDIN Research to estimate the market size.
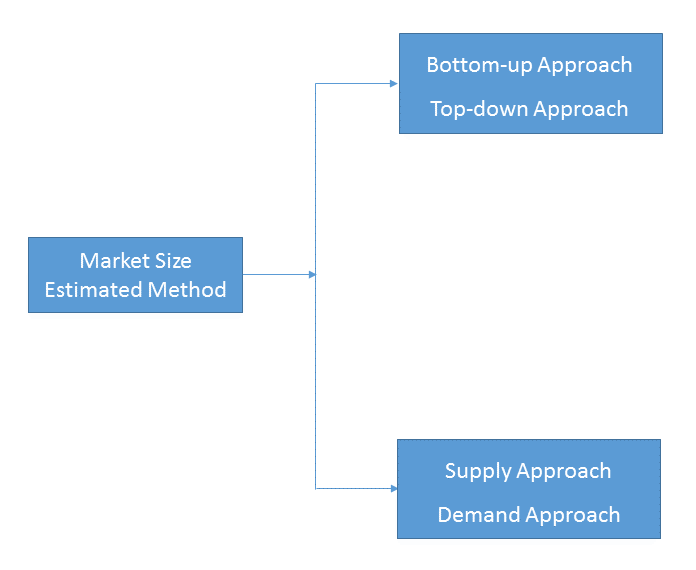
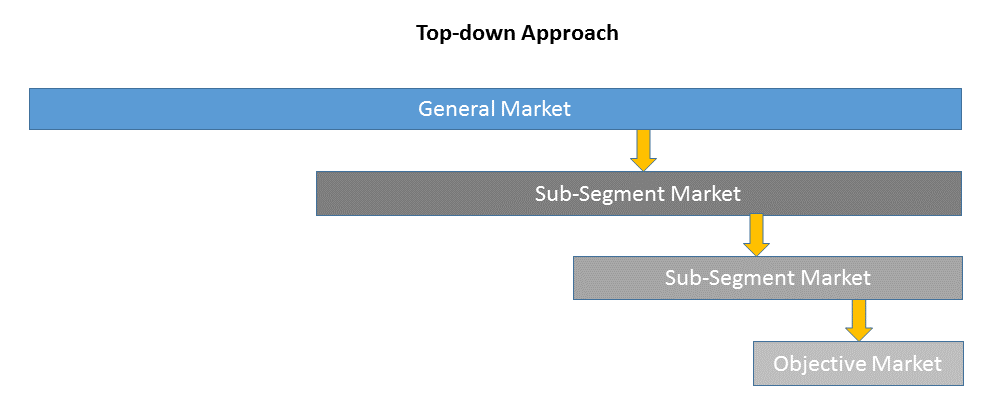
1)Top-down & Bottom-up Approach
Top-down approach uses a general market size figure and determines the percentage that the objective market represents.
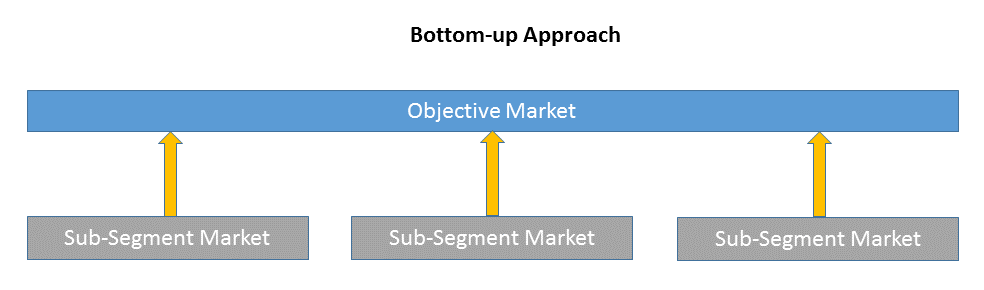
Bottom-up approach size the objective market by collecting the sub-segment information.
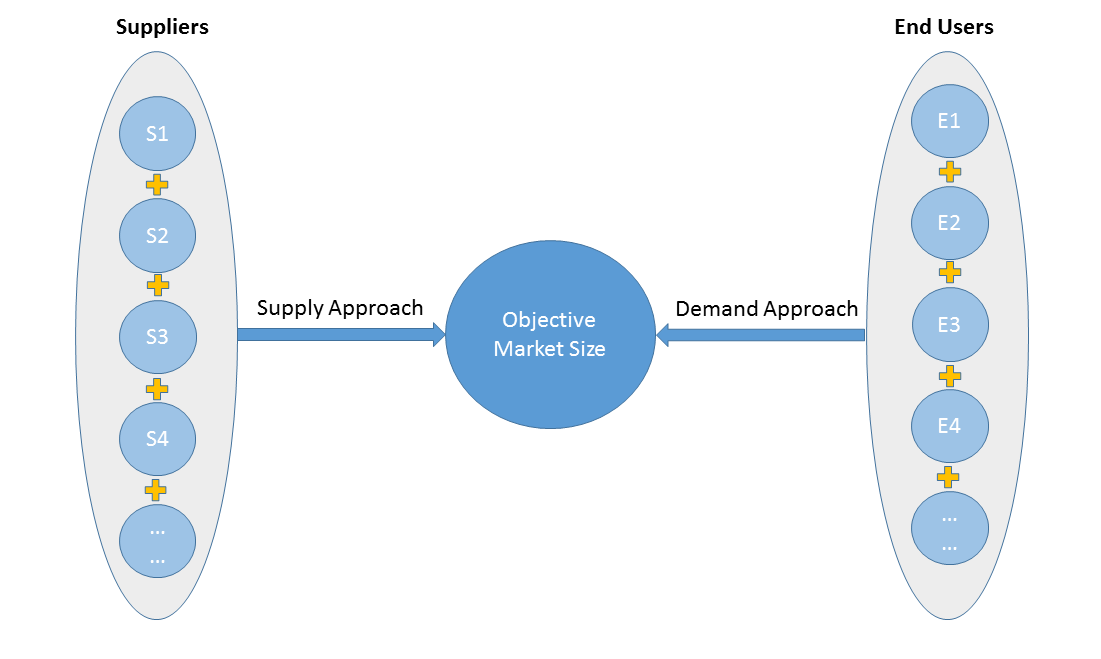
2)Supply & Demand Approach
Supply approach is based on assessments of the size of each competitor supplying the objective market.
Demand approach combine end-user data within a market to estimate the objective market size. It is sometimes referred to as bottom-up approach.
- Forecasting Methodology
- Numerous factors impacting the market trend are considered for forecast model:
- New technology and application in the future;
- New project planned/under contraction;
- Global and regional underlying economic growth;
- Threatens of substitute products;
- Industry expert opinion;
- Policy and Society implication.
- Analysis Tools
1)PEST Analysis
PEST Analysis is a simple and widely used tool that helps our client analyze the Political, Economic, Socio-Cultural, and Technological changes in their business environment.
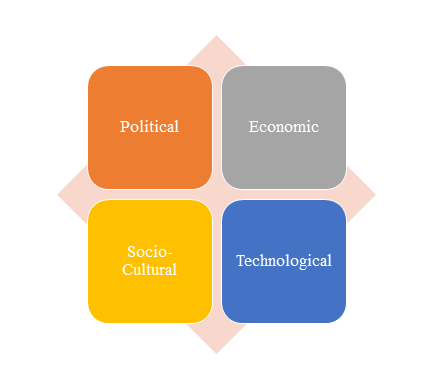
- Benefits of a PEST analysis:
- It helps you to spot business opportunities, and it gives you advanced warning of significant threats.
- It reveals the direction of change within your business environment. This helps you shape what you’re doing, so that you work with change, rather than against it.
- It helps you avoid starting projects that are likely to fail, for reasons beyond your control.
- It can help you break free of unconscious assumptions when you enter a new country, region, or market; because it helps you develop an objective view of this new environment.
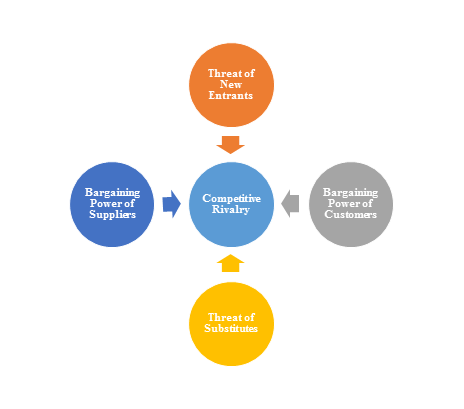
2)Porter’s Five Force Model Analysis
The Porter’s Five Force Model is a tool that can be used to analyze the opportunities and overall competitive advantage. The five forces that can assist in determining the competitive intensity and potential attractiveness within a specific area.
- Threat of New Entrants: Profitable industries that yield high returns will attract new firms.
- Threat of Substitutes: A substitute product uses a different technology to try to solve the same economic need.
- Bargaining Power of Customers: the ability of customers to put the firm under pressure, which also affects the customer's sensitivity to price changes.
- Bargaining Power of Suppliers: Suppliers of raw materials, components, labor, and services (such as expertise) to the firm can be a source of power over the firm when there are few substitutes.
- Competitive Rivalry: For most industries the intensity of competitive rivalry is the major determinant of the competitiveness of the industry.
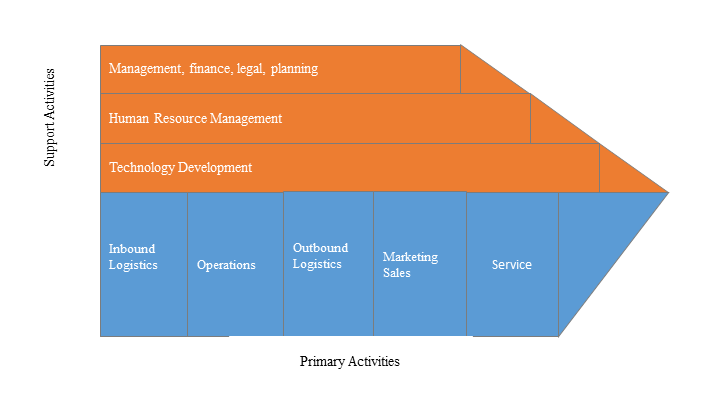
3)Value Chain Analysis
Value chain analysis is a tool to identify activities, within and around the firm and relating these activities to an assessment of competitive strength. Value chain can be analyzed by primary activities and supportive activities. Primary activities include: inbound logistics, operations, outbound logistics, marketing & sales, service. Support activities include: technology development, human resource management, management, finance, legal, planning.
4)SWOT Analysis
SWOT analysis is a tool used to evaluate a company's competitive position by identifying its strengths, weaknesses, opportunities and threats. The strengths and weakness is the inner factor; the opportunities and threats are the external factor. By analyzing the inner and external factors, the analysis can provide the detail information of the position of a player and the characteristics of the industry.
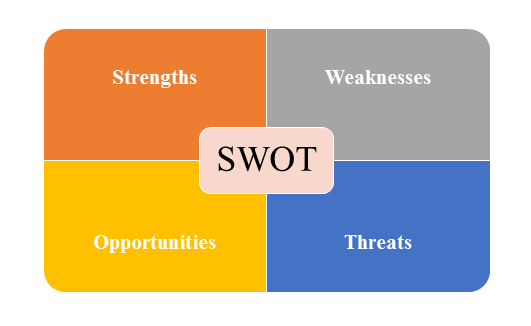
- Strengths describe what the player excels at and separates it from the competition
- Weaknesses stop the player from performing at its optimum level.
- Opportunities refer to favorable external factors that the player can use to give it a competitive advantage.
- Threats refer to factors that have the potential to harm the player.
- Data Sources
| Primary Sources | Secondary Sources |
|---|---|
| Face to face/Phone Interviews with market participants, such as: Manufactures; Distributors; End-users; Experts. Online Survey |
Government/International Organization Data: Annual Report/Presentation/Fact Book Internet Source Information Industry Association Data Free/Purchased Database Market Research Report Book/Journal/News |